The Global Point-of-care Diagnostics Market Outlook is promising, expected to reach approximately USD 82.78 billion by 2034. CAR-TOOL.EDU.VN can help you navigate this dynamic market with detailed information on various point-of-care diagnostic tools, testing kits, and market trends. By understanding these trends, professionals can make informed decisions about diagnostic equipment and improve service quality.
Contents
- 1. Understanding the Global Point-of-Care Diagnostics Market Outlook
- 1.1 Key Segments in Point-of-Care Diagnostics Market
- 1.2 Geographical Insights into Point-of-Care Diagnostics
- 2. Factors Driving Growth in the Point-of-Care Diagnostics Market
- 2.1 Technological Advancements and Microfluidics
- 2.2 Rise in Infectious Diseases and Glucose Monitoring
- 3. Applications of Point-of-Care Diagnostics
- 4. Key Players in the Point-of-Care Diagnostics Market
- 5. Regional Analysis of the Point-of-Care Diagnostics Market
- 6. Challenges and Opportunities in the Point-of-Care Diagnostics Market
- 7. Trends Shaping the Future of Point-of-Care Diagnostics
- 8. Impact of COVID-19 on the Point-of-Care Diagnostics Market
- 9. How CAR-TOOL.EDU.VN Helps You Navigate the Point-of-Care Diagnostics Market
- 10. Expert Insights and Recommendations for Point-of-Care Diagnostics
- FAQ Section: Your Questions About Point-of-Care Diagnostics Answered
- What is Point-of-Care Diagnostics (POCD)?
- What are the benefits of using Point-of-Care Diagnostics?
- What types of tests can be performed using Point-of-Care Diagnostics?
- Where are Point-of-Care Diagnostics typically used?
- How accurate are Point-of-Care Diagnostics tests?
- What are the key factors driving the growth of the Point-of-Care Diagnostics market?
- What are the challenges facing the Point-of-Care Diagnostics market?
- How does the regulatory landscape impact the Point-of-Care Diagnostics market?
- What role does technology play in the advancement of Point-of-Care Diagnostics?
- How can I stay updated on the latest trends and developments in the Point-of-Care Diagnostics market?
1. Understanding the Global Point-of-Care Diagnostics Market Outlook
What does the global point-of-care diagnostics market outlook entail? The global point-of-care diagnostics market outlook encompasses the analysis and projection of the market’s performance, trends, and opportunities related to diagnostic testing performed near the patient rather than in a central laboratory. This includes assessing market size, growth rate, technological advancements, regulatory landscape, and competitive dynamics. This analysis is crucial for understanding how point-of-care diagnostics are evolving and impacting healthcare delivery worldwide.
Point-of-care diagnostics (POCD) refers to medical diagnostic testing performed outside of a traditional laboratory setting, typically near or at the site of patient care. This approach enables rapid and convenient testing, providing quick results that can inform immediate clinical decisions. The market outlook involves understanding current market size, growth drivers, and potential future trends. According to a report by Precedence Research, the global point-of-care diagnostics market was valued at USD 62.28 billion in 2024 and is projected to reach around USD 82.78 billion by 2034, growing at a CAGR of 2.89% from 2025 to 2034.
This growth is attributed to several factors, including the increasing prevalence of chronic diseases, advancements in diagnostic technologies, and the rising demand for rapid and convenient testing solutions.
1.1 Key Segments in Point-of-Care Diagnostics Market
What are the key segments in the point-of-care diagnostics market? The key segments include product type (glucose monitoring, infectious diseases, cardiometabolic diseases, pregnancy and fertility testing, hematology testing, etc.), end-user (hospital bedside, physician’s office lab, urgent care and retail clinics, homecare/self-testing), and geography (North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa).
Understanding these segments helps in identifying specific opportunities and trends within the market.
- Product Type:
- Glucose Monitoring: Essential for managing diabetes, this segment benefits from continuous technological advancements.
- Infectious Diseases: Driven by the need for rapid detection of pathogens, especially during pandemics.
- Cardiometabolic Diseases: Includes tests for cardiac markers and lipid profiles, crucial for managing cardiovascular health.
- Pregnancy & Infertility Testing: Offers convenient and quick results, enhancing accessibility for users.
- Hematology Testing: Provides immediate blood analysis, aiding in diagnosing various blood disorders.
- End-User:
- Hospital Bedside: Facilitates immediate diagnostic results, enabling quick clinical decisions.
- Physician’s Office Lab: Enhances efficiency in primary care settings, improving patient management.
- Urgent Care & Retail Clinics: Offers accessible diagnostic services, reducing wait times and improving convenience.
- Homecare/Self-Testing: Empowers patients to monitor their health conditions from the comfort of their homes.
1.2 Geographical Insights into Point-of-Care Diagnostics
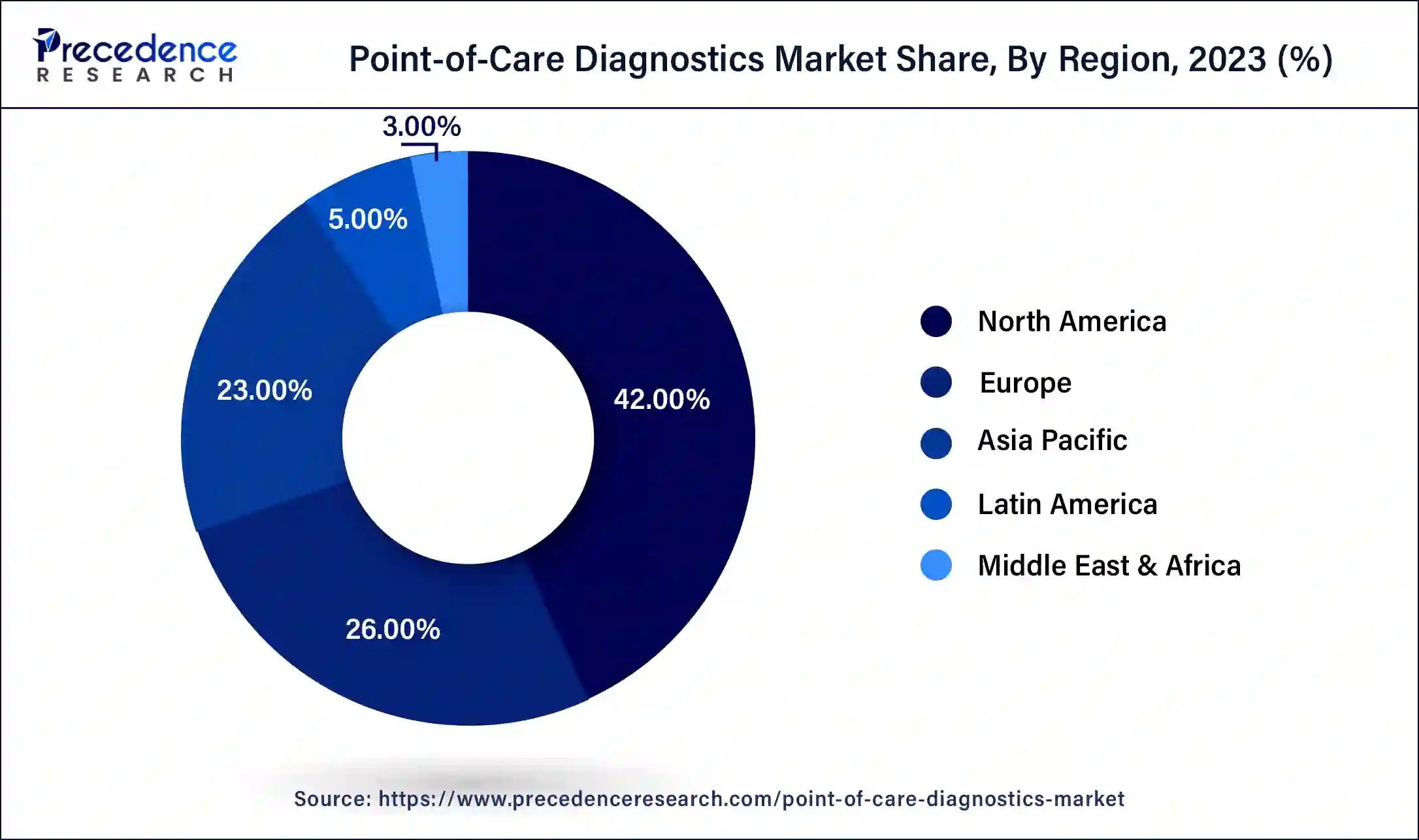
Which regions are leading the point-of-care diagnostics market? North America currently leads the market, holding 42% of the total revenue share in 2024, owing to the rising incidence of lifestyle diseases and greater awareness of self-testing. However, the Asia-Pacific region is expected to be the fastest-growing segment in the near future, driven by increasing market players, rising healthcare expenditure, and growing adoption of POCD kits in countries like China, Japan, and India.
Understanding the geographical dynamics helps businesses tailor their strategies to specific regional needs and growth potentials.
- North America:
- Driven by advanced healthcare infrastructure and high adoption rates of new technologies.
- Significant investments in R&D and favorable reimbursement policies.
- Europe:
- Stringent regulatory framework ensuring high-quality diagnostic services.
- Growing emphasis on decentralized healthcare and patient-centric approaches.
- Asia-Pacific:
- Rapidly expanding healthcare infrastructure and increasing awareness among the population.
- Rising prevalence of chronic diseases and government initiatives to improve healthcare access.
 Global Point-of-Care Diagnostics Market
Global Point-of-Care Diagnostics Market
2. Factors Driving Growth in the Point-of-Care Diagnostics Market
What factors are propelling the point-of-care diagnostics market growth? Several factors are driving the growth of the point-of-care diagnostics market, including technological advancements in POC devices, rising incidence of infectious diseases and chronic conditions, increased healthcare expenditure, and a growing preference for rapid and convenient testing solutions. The shift towards patient-centric healthcare and the need for timely clinical decisions further fuel market expansion.
The growth is significantly influenced by the increasing prevalence of chronic and infectious diseases, particularly in developing economies. According to the World Health Organization (WHO), chronic diseases such as diabetes, cardiovascular diseases, and cancer are on the rise globally due to factors like aging populations, unhealthy lifestyles, and environmental influences.
2.1 Technological Advancements and Microfluidics
How do technological advancements impact point-of-care diagnostics? Technological advancements, particularly in microfluidics, have revolutionized point-of-care diagnostics. Microfluidics enables the creation of compact, portable, and easy-to-use POC devices that can deliver rapid and accurate results. The integration of microfluidics with nanodiagnostics and lab-on-a-chip technologies further enhances the sensitivity and specificity of POC tests, making them more effective in diagnosing a wide range of diseases.
Microfluidics technology has advanced significantly in recent years, allowing for the cost-effective production of POC devices that are easy to use, portable, and capable of delivering quick results. This technology uses tiny channels to manipulate small fluid volumes, reducing reagent consumption, and enabling high-throughput analysis. The development of microfluidic devices has also facilitated the integration of multiple diagnostic tests into a single platform, enhancing the efficiency and convenience of POC testing.
2.2 Rise in Infectious Diseases and Glucose Monitoring
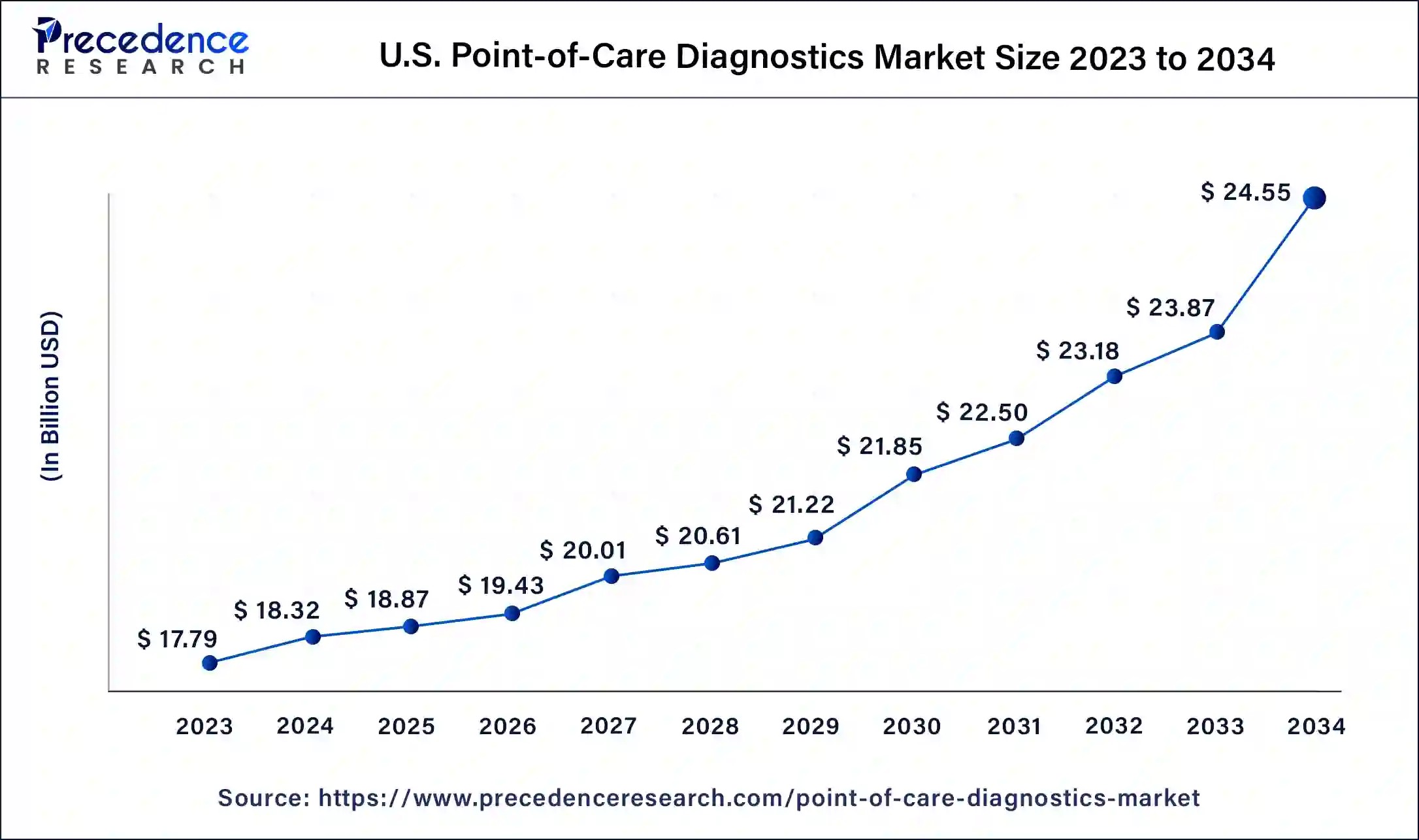
What role do infectious diseases and glucose monitoring play in market growth? The rising incidence of infectious diseases such as tuberculosis, HIV/AIDS, and influenza, as well as the frequent outbreaks of novel pathogens, has increased the demand for rapid and accurate diagnostic tests. POC tests enable quick detection of these infections, facilitating timely treatment and preventing further spread. Additionally, the increasing prevalence of diabetes and the need for continuous glucose monitoring have driven the adoption of glucose testing kits, contributing significantly to market growth.
The adoption of glucose monitoring testing kits is driven by the necessity for early detection of hyperglycemic and hypoglycemic diabetes, the high prevalence of diabetes, the convenience of continuous glucose monitoring, and growing technological innovations. According to the Centers for Disease Control and Prevention (CDC), approximately 37.3 million Americans have diabetes, and early detection and management are crucial for preventing complications.
 U.S. Point-of-Care Diagnostics Market Size
U.S. Point-of-Care Diagnostics Market Size
3. Applications of Point-of-Care Diagnostics
What are the primary applications of point-of-care diagnostics? The primary applications of point-of-care diagnostics span a wide array of medical fields, including infectious disease testing, glucose monitoring, cardiac marker detection, pregnancy testing, and coagulation monitoring. These applications are crucial in emergency rooms, intensive care units, primary care settings, and home healthcare, providing immediate results that enhance patient care and management.
The versatility of POC diagnostics makes it an indispensable tool in modern healthcare, enabling faster diagnosis, better patient outcomes, and more efficient healthcare delivery.
- Infectious Disease Testing:
- Rapid detection of pathogens such as influenza, COVID-19, and STIs.
- Enables timely isolation and treatment, preventing further spread of infections.
- Glucose Monitoring:
- Essential for managing diabetes by monitoring blood glucose levels.
- Helps patients adjust medication and lifestyle to maintain optimal glucose control.
- Cardiac Marker Detection:
- Detects cardiac markers like troponin to diagnose acute myocardial infarction.
- Enables quick intervention, reducing mortality and morbidity rates.
- Pregnancy Testing:
- Provides convenient and rapid confirmation of pregnancy.
- Allows early prenatal care, improving maternal and infant health outcomes.
- Coagulation Monitoring:
- Monitors blood clotting parameters to manage anticoagulant therapy.
- Ensures safe and effective use of anticoagulants, preventing bleeding and thrombotic events.
4. Key Players in the Point-of-Care Diagnostics Market
Who are the major companies in the point-of-care diagnostics market? The major players in the point-of-care diagnostics market include companies like Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers, Johnson & Johnson, and Danaher Corporation. These companies invest heavily in research and development, product innovation, and strategic collaborations to maintain their competitive edge and expand their market presence.
These companies are at the forefront of developing and marketing POC diagnostic solutions, driving innovation and shaping the future of the market.
- Abbott Laboratories:
- Offers a wide range of POC diagnostic solutions, including glucose monitoring systems and infectious disease tests.
- Focuses on innovation and expanding its product portfolio through strategic acquisitions.
- F. Hoffmann-La Roche Ltd:
- Provides advanced POC diagnostic platforms and assays for various clinical applications.
- Committed to developing high-quality and reliable diagnostic solutions.
5. Regional Analysis of the Point-of-Care Diagnostics Market
Which regions offer the most significant opportunities in the point-of-care diagnostics market? North America currently dominates the point-of-care diagnostics market, driven by a well-established healthcare infrastructure, high adoption of advanced technologies, and the presence of major market players. However, the Asia-Pacific region is poised to witness the highest growth rate in the coming years, owing to increasing healthcare expenditure, rising awareness about POC diagnostics, and growing prevalence of chronic diseases.
Understanding regional dynamics is crucial for businesses looking to expand their market presence and capitalize on emerging opportunities.
- North America:
- Advanced healthcare infrastructure and high adoption rates of new technologies.
- Significant investments in R&D and favorable reimbursement policies.
- Europe:
- Stringent regulatory framework ensuring high-quality diagnostic services.
- Growing emphasis on decentralized healthcare and patient-centric approaches.
- Asia-Pacific:
- Rapidly expanding healthcare infrastructure and increasing awareness among the population.
- Rising prevalence of chronic diseases and government initiatives to improve healthcare access.
6. Challenges and Opportunities in the Point-of-Care Diagnostics Market
What challenges and opportunities exist in the point-of-care diagnostics market? While the point-of-care diagnostics market offers significant growth opportunities, it also faces certain challenges. These challenges include stringent regulatory requirements, concerns about data security and connectivity, and the need for standardization and quality control. However, the market also presents numerous opportunities, such as the development of innovative POC testing solutions, expansion into emerging markets, and integration of POC diagnostics with telehealth and remote monitoring platforms.
Addressing these challenges and capitalizing on the opportunities will be crucial for sustaining growth and realizing the full potential of POC diagnostics.
- Challenges:
- Regulatory Requirements: Ensuring compliance with stringent regulations to maintain quality and safety.
- Data Security and Connectivity: Protecting patient data and ensuring seamless integration with healthcare IT systems.
- Opportunities:
- Innovative POC Testing Solutions: Developing advanced tests for a wider range of diseases and conditions.
- Expansion into Emerging Markets: Tapping into the growth potential of developing economies with increasing healthcare needs.
7. Trends Shaping the Future of Point-of-Care Diagnostics
What are the emerging trends in the point-of-care diagnostics market? Several key trends are shaping the future of the point-of-care diagnostics market. These include the increasing adoption of connected POC devices, the integration of artificial intelligence (AI) and machine learning (ML) in diagnostic testing, the development of multiplexed assays, and the growing focus on personalized medicine. These trends are expected to drive further innovation and transform the way healthcare is delivered.
Staying abreast of these trends is essential for stakeholders looking to stay ahead of the curve and capitalize on emerging opportunities.
- Connected POC Devices:
- Enables real-time data transmission and remote monitoring, improving patient management.
- Facilitates data-driven decision-making and enhances healthcare efficiency.
- AI and Machine Learning:
- Enhances diagnostic accuracy and enables predictive analysis.
- Streamlines workflows and reduces the risk of human error.
- Multiplexed Assays:
- Allows simultaneous detection of multiple analytes, improving diagnostic efficiency.
- Reduces the need for multiple tests, saving time and resources.
- Personalized Medicine:
- Tailors diagnostic and treatment decisions to individual patient characteristics.
- Improves patient outcomes and reduces the risk of adverse effects.
8. Impact of COVID-19 on the Point-of-Care Diagnostics Market
How did the COVID-19 pandemic affect the point-of-care diagnostics market? The COVID-19 pandemic has had a significant impact on the point-of-care diagnostics market, leading to a surge in demand for rapid and accurate diagnostic tests. POC tests have played a crucial role in managing the pandemic by enabling quick detection of the virus, facilitating timely isolation and treatment, and preventing further spread. The pandemic has also accelerated the development and adoption of innovative POC testing solutions, driving market growth and transforming the diagnostic landscape.
The pandemic has highlighted the importance of POC diagnostics in managing public health crises and has paved the way for future growth and innovation in the market.
- Increased Demand for Rapid Testing:
- POC tests enabled quick detection of COVID-19, facilitating timely intervention.
- Reduced the burden on central laboratories and improved testing accessibility.
- Accelerated Innovation:
- Spurred the development of novel POC testing solutions and technologies.
- Enhanced the efficiency and accuracy of diagnostic testing.
9. How CAR-TOOL.EDU.VN Helps You Navigate the Point-of-Care Diagnostics Market
How can CAR-TOOL.EDU.VN assist in understanding the point-of-care diagnostics market? CAR-TOOL.EDU.VN provides detailed information and comparisons of various point-of-care diagnostic tools and testing kits, aiding professionals in making informed decisions. The platform offers comprehensive specifications, user reviews, and expert opinions to help users assess the durability, effectiveness, and value of different diagnostic equipment. This resource is essential for those looking to enhance their diagnostic capabilities.
By offering this information, CAR-TOOL.EDU.VN ensures that automotive service providers can select the best tools to meet their specific needs, ultimately improving the quality and efficiency of their services.
- Detailed Product Information:
- Comprehensive specifications for various diagnostic tools and testing kits.
- Helps in comparing features and performance of different products.
- User Reviews and Expert Opinions:
- Provides insights from real users and industry experts.
- Aids in assessing the reliability and effectiveness of diagnostic equipment.
10. Expert Insights and Recommendations for Point-of-Care Diagnostics
What expert insights and recommendations can guide decisions in the point-of-care diagnostics market? Experts recommend that healthcare providers prioritize accuracy, reliability, and ease of use when selecting point-of-care diagnostic tools. Additionally, they emphasize the importance of adhering to quality control measures and ensuring proper training for personnel operating POC devices. For automotive professionals looking to integrate diagnostic tools, understanding the specific vehicle systems and diagnostic requirements is crucial.
These recommendations are essential for maximizing the benefits of POC diagnostics and ensuring optimal patient outcomes.
- Prioritize Accuracy and Reliability:
- Select POC devices that offer high sensitivity and specificity.
- Ensure compliance with quality control standards to maintain accuracy.
- Ensure Proper Training:
- Provide comprehensive training for personnel operating POC devices.
- Regularly update training to reflect advancements in technology and best practices.
Navigating the point-of-care diagnostics market requires a blend of expertise and access to reliable information. By understanding the market’s dynamics, key drivers, and emerging trends, professionals can make informed decisions that enhance patient care and improve healthcare outcomes. CAR-TOOL.EDU.VN serves as a valuable resource in this journey, offering detailed insights and practical guidance to help you navigate the point-of-care diagnostics market effectively.
Are you looking for detailed information on specific point-of-care diagnostic tools or need assistance in comparing different options? Contact CAR-TOOL.EDU.VN today at 456 Elm Street, Dallas, TX 75201, United States, or reach us via WhatsApp at +1 (641) 206-8880 for expert guidance and support. Let us help you enhance your diagnostic capabilities and improve the quality of your services.
FAQ Section: Your Questions About Point-of-Care Diagnostics Answered
What is Point-of-Care Diagnostics (POCD)?
Point-of-Care Diagnostics (POCD) refers to medical diagnostic testing performed near or at the site of patient care, outside of a traditional laboratory setting. This approach enables rapid and convenient testing, providing quick results that can inform immediate clinical decisions. POCD encompasses a range of tests and technologies designed to deliver timely and actionable diagnostic information.
What are the benefits of using Point-of-Care Diagnostics?
The benefits of using Point-of-Care Diagnostics are numerous, including faster turnaround times for test results, improved patient outcomes, reduced healthcare costs, and enhanced patient satisfaction. POCD enables timely clinical decisions, facilitates early intervention, and improves access to diagnostic testing in remote or resource-limited settings. These benefits make POCD an essential tool in modern healthcare delivery.
What types of tests can be performed using Point-of-Care Diagnostics?
Many types of tests can be performed using Point-of-Care Diagnostics, including glucose monitoring, infectious disease testing (e.g., influenza, COVID-19), cardiac marker detection, pregnancy testing, coagulation monitoring, and blood gas analysis. The versatility of POCD allows for the rapid and convenient assessment of various health conditions, aiding in diagnosis and treatment decisions.
Where are Point-of-Care Diagnostics typically used?
Point-of-Care Diagnostics are typically used in various healthcare settings, including hospitals (e.g., emergency rooms, intensive care units), physician’s offices, urgent care centers, retail clinics, and home healthcare. The portability and ease of use of POCD devices make them suitable for use in diverse environments, bringing diagnostic testing closer to the patient.
How accurate are Point-of-Care Diagnostics tests?
Point-of-Care Diagnostics tests are generally accurate, but their accuracy can vary depending on the type of test, the technology used, and the quality control measures in place. It’s essential to select POCD devices that have been validated for accuracy and reliability, and to ensure that personnel operating the devices are properly trained. Regular quality control checks are also necessary to maintain accuracy over time.
What are the key factors driving the growth of the Point-of-Care Diagnostics market?
The key factors driving the growth of the Point-of-Care Diagnostics market include the increasing prevalence of chronic and infectious diseases, the growing demand for rapid and convenient testing solutions, technological advancements in POCD devices, and the shift towards patient-centric healthcare. These factors contribute to the expansion of the POCD market and the increasing adoption of POCD technologies.
What are the challenges facing the Point-of-Care Diagnostics market?
The challenges facing the Point-of-Care Diagnostics market include stringent regulatory requirements, concerns about data security and connectivity, the need for standardization and quality control, and the cost of POCD devices and tests. Addressing these challenges is crucial for sustaining growth and realizing the full potential of POCD in healthcare.
How does the regulatory landscape impact the Point-of-Care Diagnostics market?
The regulatory landscape significantly impacts the Point-of-Care Diagnostics market, as POCD devices and tests are subject to regulatory oversight by agencies such as the FDA in the United States and the EMA in Europe. Compliance with regulatory requirements is essential for ensuring the safety, efficacy, and quality of POCD products, and for gaining market access.
What role does technology play in the advancement of Point-of-Care Diagnostics?
Technology plays a critical role in the advancement of Point-of-Care Diagnostics, driving innovation in POCD devices, testing methodologies, and data management systems. Technological advancements such as microfluidics, nanodiagnostics, and connectivity solutions enable the development of more accurate, convenient, and efficient POCD tests, enhancing their utility in healthcare settings.
How can I stay updated on the latest trends and developments in the Point-of-Care Diagnostics market?
You can stay updated on the latest trends and developments in the Point-of-Care Diagnostics market by following industry news and publications, attending conferences and trade shows, subscribing to newsletters and alerts from market research firms, and consulting with experts in the field. Platforms like CAR-TOOL.EDU.VN offer valuable insights and resources to help you stay informed about the POCD market.