Synthetic Biology-based Point-of-care Diagnostics For Infectious Disease offers rapid, accessible, and precise detection of pathogens, transforming healthcare accessibility as highlighted by CAR-TOOL.EDU.VN. These innovative diagnostics leverage synthetic biology to create user-friendly, portable devices that can quickly identify infectious diseases at the point of care. Explore the potential of bio-detection tools, molecular diagnostics, and ambient nucleic acid amplification to revolutionize disease detection and management.
Contents
- 1. Understanding Synthetic Biology in Point-of-Care Diagnostics
- 1.1. What Role Does Synthetic Biology Play in Revolutionizing Point-of-Care Diagnostics?
- 1.2. How Does Synthetic Biology Enhance the Specificity and Sensitivity of Diagnostic Tests?
- Specificity Enhancement
- Sensitivity Enhancement
- 1.3. What Are the Key Synthetic Biology Techniques Used in Diagnostics?
- 2. Point-of-Care Diagnostics: A Closer Look
- 2.1. What Defines Point-of-Care Diagnostics and Why Is It Important?
- 2.2. What Are the Advantages and Limitations of Point-of-Care Diagnostic Tools?
- Advantages
- Limitations
- 2.3. What Types of Infectious Diseases Can Be Diagnosed Using Point-of-Care Tests?
- 3. The Intersection of Synthetic Biology and Point-of-Care Diagnostics
- 3.1. How Does Combining Synthetic Biology with Point-of-Care Diagnostics Improve Disease Detection?
- 3.2. What Are Some Examples of Synthetic Biology-Based Point-of-Care Diagnostic Devices?
- 3.3. What Challenges Need to Be Addressed to Broaden the Use of These Diagnostics?
- 4. Applications in Infectious Disease Detection
- 4.1. How Can Synthetic Biology-Based Point-of-Care Diagnostics Be Used to Combat Pandemics?
- 4.2. What Role Can These Diagnostics Play in Resource-Limited Settings?
- 4.3. How Do These Diagnostics Improve Patient Outcomes in Infectious Disease Management?
- 5. Overcoming Challenges and Future Directions
- 5.1. What Are the Main Obstacles to the Widespread Adoption of Synthetic Biology Diagnostics?
- 5.2. How Can These Obstacles Be Overcome?
- 5.3. What Are the Future Directions for Synthetic Biology-Based Point-of-Care Diagnostics?
- FAQ: Synthetic Biology-Based Point-of-Care Diagnostics for Infectious Disease
- Q1: What are synthetic biology-based point-of-care diagnostics?
- Q2: How do synthetic biology diagnostics differ from traditional diagnostic methods?
- Q3: What types of infectious diseases can be diagnosed using these diagnostics?
- Q4: What are the advantages of using point-of-care diagnostics in resource-limited settings?
- Q5: How can synthetic biology diagnostics help in combating pandemics?
- Q6: What are the main challenges in the widespread adoption of synthetic biology diagnostics?
- Q7: How are researchers addressing the challenges of stability and shelf life in these diagnostics?
- Q8: What role does artificial intelligence play in the future of synthetic biology diagnostics?
- Q9: Are there any ethical concerns associated with the use of synthetic biology in diagnostics?
- Q10: How can I learn more about synthetic biology and point-of-care diagnostics?
1. Understanding Synthetic Biology in Point-of-Care Diagnostics
1.1. What Role Does Synthetic Biology Play in Revolutionizing Point-of-Care Diagnostics?
Synthetic biology plays a pivotal role in revolutionizing point-of-care diagnostics by enabling the creation of highly specific, sensitive, and rapid diagnostic tools for infectious diseases. According to a study by the National Institutes of Health (NIH) in 2022, synthetic biology integrates engineering principles with biology to design and construct new biological parts, devices, and systems, or to redesign existing natural biological systems for useful purposes. This field facilitates the development of biosensors and diagnostic assays that can detect pathogens or disease biomarkers directly at the point of care, eliminating the need for centralized laboratory testing.
Synthetic biology enhances the capabilities of point-of-care diagnostics through several key mechanisms:
- Enhanced Specificity: Synthetic biology allows for the design of highly specific recognition elements, such as engineered proteins or nucleic acids, that can selectively bind to target molecules unique to a particular pathogen.
- Improved Sensitivity: By incorporating amplification strategies, such as nucleic acid amplification or signal amplification cascades, synthetic biology-based diagnostics can detect even trace amounts of a pathogen, crucial for early diagnosis.
- Rapid Response Times: Synthetic biology enables the creation of fast-acting diagnostic systems that can provide results in minutes, facilitating timely clinical decisions and interventions.
- Portability and Ease of Use: Synthetic biology-based devices are often designed to be portable, user-friendly, and require minimal training, making them suitable for deployment in resource-limited settings.
- Versatility: Synthetic biology can be used to develop diagnostics for a wide range of infectious diseases, from viral infections like COVID-19 to bacterial infections like tuberculosis.
The integration of synthetic biology into point-of-care diagnostics represents a paradigm shift in healthcare, enabling faster, more accessible, and more effective disease detection and management. CAR-TOOL.EDU.VN emphasizes the importance of these advancements in transforming healthcare accessibility.
1.2. How Does Synthetic Biology Enhance the Specificity and Sensitivity of Diagnostic Tests?
Synthetic biology significantly enhances the specificity and sensitivity of diagnostic tests through the engineering of biological systems that can precisely detect and amplify signals from target pathogens. A 2021 study in “Nature Chemical Biology” highlights how synthetic biology tools are used to create biosensors with tailored recognition elements and amplification mechanisms, improving diagnostic accuracy.
Specificity Enhancement
- Engineered Binding Proteins: Synthetic biology allows for the design of proteins that specifically bind to unique biomarkers of a pathogen. For example, synthetic antibodies or aptamers can be engineered to recognize surface proteins of a virus, ensuring that the diagnostic test only reacts in the presence of the target pathogen.
- Customized Nucleic Acid Probes: Synthetic DNA or RNA probes can be designed to hybridize with specific sequences in the pathogen’s genome. These probes can be tailored to distinguish between closely related strains, minimizing false positives.
Sensitivity Enhancement
- Nucleic Acid Amplification Techniques: Synthetic biology facilitates the integration of highly efficient nucleic acid amplification methods, such as PCR, LAMP, or CRISPR-based techniques, into diagnostic tests. These methods exponentially increase the amount of target DNA or RNA, making it detectable even when present in low concentrations.
- Signal Amplification Cascades: Synthetic biology can create intricate biological circuits that amplify the initial detection signal. For instance, enzyme-linked reactions can be designed to produce a detectable output signal proportional to the amount of the target pathogen present.
- Cell-Based Biosensors: Engineered cells can be used as biosensors to detect specific pathogens. When the target pathogen is detected, the cells can be programmed to produce a detectable signal, such as fluorescence or bioluminescence, amplifying the detection event.
1.3. What Are the Key Synthetic Biology Techniques Used in Diagnostics?
Several key synthetic biology techniques are used in diagnostics, each contributing unique capabilities to enhance the detection and management of infectious diseases. A comprehensive review in “Biosensors and Bioelectronics” (2023) outlines the most impactful techniques:
- CRISPR-Based Diagnostics: CRISPR-Cas systems have revolutionized diagnostics by providing highly specific and sensitive detection of nucleic acids. CRISPR-based diagnostics, such as SHERLOCK and DETECTR, can quickly identify pathogens by targeting their DNA or RNA with engineered guide RNAs and Cas enzymes.
- Nucleic Acid Aptamers: Aptamers are synthetic oligonucleotides that bind to specific target molecules with high affinity. They can be used as recognition elements in biosensors to detect a wide range of pathogens, including viruses, bacteria, and parasites.
- Engineered Enzymes and Proteins: Synthetic biology allows for the design of enzymes and proteins with enhanced catalytic activity, stability, and specificity. These engineered proteins can be used in diagnostic assays to amplify signals or to detect specific biomarkers of infection.
- Cell-Free Synthetic Biology: Cell-free systems, which contain the essential components for gene expression without living cells, offer a versatile platform for rapid and portable diagnostics. Cell-free systems can be programmed to produce detectable signals in response to specific pathogens, enabling point-of-care testing.
- Microfluidics and Lab-on-a-Chip Devices: Synthetic biology is often integrated with microfluidics and lab-on-a-chip technologies to create miniaturized diagnostic devices. These devices can automate complex diagnostic assays, reduce reagent consumption, and provide rapid results.
- DNA/RNA Synthesis: Synthetic biology employs advanced techniques for synthesizing DNA and RNA sequences, enabling the creation of customized probes, primers, and guide RNAs for diagnostic applications.
- Directed Evolution: Directed evolution is used to optimize the properties of proteins and enzymes for diagnostic applications. By subjecting proteins to iterative rounds of mutation and selection, researchers can improve their affinity, specificity, and stability.
These techniques are continually evolving, driving innovation in diagnostic capabilities and paving the way for more effective and accessible healthcare solutions.
2. Point-of-Care Diagnostics: A Closer Look
2.1. What Defines Point-of-Care Diagnostics and Why Is It Important?
Point-of-care diagnostics (POCD) are diagnostic tests performed near or at the site of patient care, providing rapid and actionable results. According to the World Health Organization (WHO), POCD is critical for improving healthcare outcomes, especially in resource-limited settings, by enabling timely clinical decisions and interventions.
POCD is characterized by several key features:
- Proximity to Patient: POCD tests are conducted close to the patient, whether in a clinic, at home, or in the field.
- Rapid Results: POCD devices deliver results quickly, often within minutes, allowing for immediate clinical decision-making.
- Ease of Use: POCD tests are designed to be user-friendly, requiring minimal training and expertise to perform accurately.
- Portability: POCD devices are typically portable and can be easily transported to different locations.
- Accessibility: POCD improves access to diagnostic testing, particularly in areas with limited healthcare infrastructure.
The importance of POCD lies in its ability to transform healthcare delivery by:
- Enabling Early Diagnosis: Rapid results facilitate early detection of diseases, leading to prompt treatment and improved patient outcomes.
- Improving Patient Management: POCD allows healthcare providers to monitor patients’ conditions in real-time, enabling personalized treatment strategies.
- Reducing Healthcare Costs: By reducing the need for centralized laboratory testing and follow-up visits, POCD can lower healthcare costs.
- Enhancing Disease Surveillance: POCD can be used for widespread screening and surveillance of infectious diseases, helping to control outbreaks and epidemics.
- Empowering Patients: POCD empowers patients to take control of their health by providing them with access to diagnostic testing and information.
2.2. What Are the Advantages and Limitations of Point-of-Care Diagnostic Tools?
Point-of-care diagnostic (POCD) tools offer numerous advantages that have transformed healthcare delivery, particularly in resource-limited settings. However, they also have certain limitations that need to be addressed. A 2022 review in “Clinical Chemistry and Laboratory Medicine” provides a balanced perspective on the benefits and drawbacks of POCD:
Advantages
- Rapid Turnaround Time: POCD devices provide results quickly, often within minutes, enabling timely clinical decisions and interventions.
- Accessibility: POCD improves access to diagnostic testing, particularly in remote or underserved areas with limited healthcare infrastructure.
- Ease of Use: POCD tests are designed to be user-friendly, requiring minimal training and expertise to perform accurately.
- Portability: POCD devices are typically portable and can be easily transported to different locations, making them suitable for field use.
- Reduced Healthcare Costs: By reducing the need for centralized laboratory testing and follow-up visits, POCD can lower healthcare costs.
- Improved Patient Outcomes: Early diagnosis and timely treatment resulting from POCD can lead to better patient outcomes and reduced morbidity and mortality.
- Enhanced Disease Surveillance: POCD can be used for widespread screening and surveillance of infectious diseases, helping to control outbreaks and epidemics.
Limitations
- Accuracy Concerns: Some POCD tests may have lower accuracy compared to laboratory-based assays, particularly for complex or multi-analyte tests.
- Quality Control Issues: Maintaining quality control in POCD settings can be challenging, especially in resource-limited environments.
- Regulatory Oversight: POCD tests may be subject to less stringent regulatory oversight compared to laboratory-based tests, raising concerns about their reliability and performance.
- Connectivity and Data Management: Lack of connectivity and data management capabilities can hinder the integration of POCD results into electronic health records and surveillance systems.
- Cost Considerations: While POCD can reduce overall healthcare costs, the initial investment in POCD devices and reagents can be a barrier for some healthcare providers.
2.3. What Types of Infectious Diseases Can Be Diagnosed Using Point-of-Care Tests?
Point-of-care tests (POCTs) have expanded the range of infectious diseases that can be diagnosed rapidly and accurately at the point of care. According to a report by the Centers for Disease Control and Prevention (CDC) in 2023, POCTs are available for a wide variety of infectious diseases, including:
- Respiratory Infections: POCTs are commonly used to diagnose respiratory infections such as influenza, respiratory syncytial virus (RSV), and Streptococcus pyogenes (strep throat). Rapid influenza diagnostic tests (RIDTs) and rapid strep tests are widely used in clinics and emergency departments.
- Sexually Transmitted Infections (STIs): POCTs are available for the diagnosis of STIs such as HIV, syphilis, chlamydia, and gonorrhea. These tests are particularly important for screening high-risk populations and for use in settings with limited access to laboratory testing.
- Gastrointestinal Infections: POCTs can be used to detect gastrointestinal pathogens such as Clostridium difficile, norovirus, and rotavirus. These tests are valuable for diagnosing and managing outbreaks in healthcare facilities and community settings.
- Bloodborne Infections: POCTs are available for the diagnosis of bloodborne infections such as malaria, dengue fever, and Zika virus. These tests are crucial for rapid diagnosis and treatment in endemic areas.
- Healthcare-Associated Infections (HAIs): POCTs can be used to detect HAIs such as methicillin-resistant Staphylococcus aureus (MRSA) and carbapenem-resistant Enterobacteriaceae (CRE). These tests help to prevent the spread of infections in healthcare settings.
- Tuberculosis (TB): POCTs such as the Xpert MTB/RIF assay are used for the rapid diagnosis of TB and detection of rifampicin resistance. These tests have significantly improved TB diagnosis and management, particularly in resource-limited settings.
- COVID-19: Rapid antigen tests and molecular tests have been developed for the point-of-care diagnosis of COVID-19. These tests have played a critical role in controlling the pandemic by enabling rapid detection and isolation of infected individuals.
3. The Intersection of Synthetic Biology and Point-of-Care Diagnostics
3.1. How Does Combining Synthetic Biology with Point-of-Care Diagnostics Improve Disease Detection?
Combining synthetic biology with point-of-care diagnostics (POCD) significantly improves disease detection by enhancing the sensitivity, specificity, and speed of diagnostic tests. A 2020 study in “ACS Synthetic Biology” highlights how synthetic biology tools can be engineered to create highly precise and rapid diagnostic systems for infectious diseases.
The synergistic benefits of this combination include:
- Enhanced Sensitivity: Synthetic biology allows for the design of biological circuits that amplify the detection signal, making it possible to detect even trace amounts of a pathogen.
- Improved Specificity: Synthetic biology enables the creation of highly specific recognition elements, such as engineered proteins or nucleic acids, that selectively bind to target molecules unique to a particular pathogen.
- Rapid Response Times: Synthetic biology-based diagnostics can be engineered to provide results in minutes, facilitating timely clinical decisions and interventions.
- Portability and Ease of Use: Synthetic biology-based devices are often designed to be portable, user-friendly, and require minimal training, making them suitable for deployment in resource-limited settings.
- Versatility: Synthetic biology can be used to develop diagnostics for a wide range of infectious diseases, from viral infections like COVID-19 to bacterial infections like tuberculosis.
- Cost-Effectiveness: Synthetic biology-based POCD can reduce the need for centralized laboratory testing, lowering healthcare costs and improving access to diagnostic testing in resource-limited settings.
- Customization: Synthetic biology allows for the rapid customization of diagnostic tests to detect emerging pathogens or to differentiate between closely related strains.
3.2. What Are Some Examples of Synthetic Biology-Based Point-of-Care Diagnostic Devices?
Several synthetic biology-based point-of-care diagnostic devices have been developed to detect infectious diseases rapidly and accurately. A review in “Lab on a Chip” (2021) highlights some notable examples:
- SHERLOCK (Specific High-Sensitivity Enzymatic Reporter UnLOCKing): Developed by Sherlock Biosciences, SHERLOCK is a CRISPR-based diagnostic platform that can detect RNA or DNA from pathogens with high sensitivity and specificity. It has been used to detect viruses such as Zika, dengue, and SARS-CoV-2.
- DETECTR (DNA Endonuclease Targeted CRISPR Trans Reporter): Developed by Mammoth Biosciences, DETECTR is another CRISPR-based diagnostic platform that can detect DNA from pathogens. It has been used to detect HPV and other infectious agents.
- Cell-Free Diagnostics: Researchers have developed cell-free diagnostic systems that can detect pathogens using synthetic biology circuits. These systems contain the essential components for gene expression without living cells, enabling rapid and portable diagnostics. For example, cell-free systems have been used to detect Zika virus and Ebola virus.
- Paper-Based Diagnostics: Synthetic biology has been used to create paper-based diagnostic tests that can detect pathogens in a simple and cost-effective manner. These tests often involve the use of engineered enzymes or proteins that produce a detectable signal in the presence of the target pathogen.
- Wearable Diagnostics: Researchers have developed wearable diagnostic devices that can continuously monitor biomarkers of infection. For example, a COVID-19-detecting face mask has been developed using synthetic biology principles.
3.3. What Challenges Need to Be Addressed to Broaden the Use of These Diagnostics?
Several challenges need to be addressed to broaden the use of synthetic biology-based point-of-care diagnostics (POCD). A 2023 perspective in “Nature Biotechnology” identifies key obstacles and potential solutions:
- Regulatory Approval: Synthetic biology-based diagnostics must undergo rigorous evaluation and approval by regulatory agencies such as the FDA and EMA. Streamlining the regulatory pathway for these innovative diagnostics is essential to accelerate their adoption.
- Scalability and Manufacturing: Scaling up the production of synthetic biology-based diagnostics can be challenging. Developing robust and cost-effective manufacturing processes is crucial to ensure widespread availability.
- Stability and Shelf Life: Many synthetic biology-based reagents and devices have limited stability and shelf life, particularly under harsh environmental conditions. Improving the stability of these products is essential for their use in resource-limited settings.
- Cost Reduction: The cost of synthetic biology-based diagnostics can be a barrier to their adoption, particularly in low-income countries. Reducing the cost of these diagnostics through process optimization and economies of scale is essential to improve access.
- User Training and Acceptance: Healthcare providers and patients need to be trained on how to use synthetic biology-based diagnostics correctly. Ensuring user acceptance and compliance is crucial for the successful implementation of these technologies.
- Data Connectivity and Integration: Integrating synthetic biology-based POCD into electronic health records and surveillance systems can be challenging. Developing robust data connectivity and integration solutions is essential to maximize the impact of these diagnostics.
- Ethical Considerations: The use of synthetic biology in diagnostics raises ethical considerations related to privacy, data security, and informed consent. Addressing these ethical issues is crucial to ensure responsible development and deployment of these technologies.
4. Applications in Infectious Disease Detection
4.1. How Can Synthetic Biology-Based Point-of-Care Diagnostics Be Used to Combat Pandemics?
Synthetic biology-based point-of-care diagnostics (POCD) can play a crucial role in combating pandemics by enabling rapid and widespread detection of infectious agents. A 2022 report by the National Academies of Sciences, Engineering, and Medicine highlights how these diagnostics can be deployed effectively during a pandemic:
- Rapid Detection: Synthetic biology-based POCD can provide results in minutes, enabling timely identification of infected individuals and prompt implementation of control measures.
- Widespread Screening: Synthetic biology-based POCD can be deployed for widespread screening of populations, helping to identify asymptomatic carriers and prevent further spread of the virus.
- Decentralized Testing: Synthetic biology-based POCD can be used in decentralized settings such as clinics, schools, and workplaces, reducing the burden on centralized laboratories and improving access to testing.
- Surveillance: Synthetic biology-based POCD can be used for continuous surveillance of viral strains, enabling early detection of emerging variants and prompt adaptation of control measures.
- Resource-Limited Settings: Synthetic biology-based POCD can be particularly valuable in resource-limited settings with limited access to laboratory infrastructure, enabling rapid diagnosis and management of infectious diseases.
- Contact Tracing: Rapid results from synthetic biology-based POCD can facilitate contact tracing efforts, helping to identify and isolate individuals who may have been exposed to the virus.
4.2. What Role Can These Diagnostics Play in Resource-Limited Settings?
Synthetic biology-based point-of-care diagnostics (POCD) are particularly valuable in resource-limited settings due to their portability, ease of use, and rapid results. A 2021 study in “PLOS Neglected Tropical Diseases” emphasizes the impact of these diagnostics in such environments:
- Accessibility: POCD improves access to diagnostic testing in remote or underserved areas with limited healthcare infrastructure.
- Rapid Results: POCD provides results quickly, often within minutes, enabling timely clinical decisions and interventions.
- Ease of Use: POCD tests are designed to be user-friendly, requiring minimal training and expertise to perform accurately.
- Portability: POCD devices are typically portable and can be easily transported to different locations, making them suitable for field use.
- Reduced Healthcare Costs: By reducing the need for centralized laboratory testing and follow-up visits, POCD can lower healthcare costs.
- Improved Patient Outcomes: Early diagnosis and timely treatment resulting from POCD can lead to better patient outcomes and reduced morbidity and mortality.
- Enhanced Disease Surveillance: POCD can be used for widespread screening and surveillance of infectious diseases, helping to control outbreaks and epidemics.
4.3. How Do These Diagnostics Improve Patient Outcomes in Infectious Disease Management?
Synthetic biology-based point-of-care diagnostics (POCD) significantly improve patient outcomes in infectious disease management through rapid detection, timely intervention, and personalized treatment strategies. A 2023 meta-analysis in “The Lancet Infectious Diseases” demonstrates the benefits of POCD in enhancing patient care:
- Early Diagnosis: POCD allows for the early detection of infectious diseases, leading to prompt treatment and improved patient outcomes.
- Timely Treatment: Rapid results from POCD enable healthcare providers to initiate appropriate treatment strategies without delay, reducing the risk of complications and mortality.
- Personalized Medicine: POCD can provide information about the specific pathogen causing the infection, allowing for targeted treatment with the most effective antibiotics or antiviral drugs.
- Antimicrobial Stewardship: By providing rapid diagnostic results, POCD can help to reduce the overuse of broad-spectrum antibiotics, contributing to antimicrobial stewardship efforts and preventing the development of antibiotic resistance.
- Improved Patient Compliance: POCD can improve patient compliance with treatment by providing immediate feedback and reducing the need for follow-up visits.
- Reduced Hospitalizations: Early diagnosis and treatment resulting from POCD can reduce the need for hospitalizations, lowering healthcare costs and improving patient comfort.
- Enhanced Disease Surveillance: POCD can be used for widespread screening and surveillance of infectious diseases, helping to control outbreaks and epidemics and protect public health.
5. Overcoming Challenges and Future Directions
5.1. What Are the Main Obstacles to the Widespread Adoption of Synthetic Biology Diagnostics?
The widespread adoption of synthetic biology diagnostics faces several significant obstacles. A 2022 report from the National Science Foundation (NSF) highlights these challenges:
- Regulatory Hurdles: Navigating the regulatory landscape for synthetic biology diagnostics can be complex and time-consuming. Clear and streamlined regulatory pathways are needed to facilitate the approval and commercialization of these technologies.
- Scalability and Manufacturing: Scaling up the production of synthetic biology diagnostics can be challenging. Developing robust and cost-effective manufacturing processes is crucial to ensure widespread availability.
- Stability and Shelf Life: Many synthetic biology-based reagents and devices have limited stability and shelf life, particularly under harsh environmental conditions. Improving the stability of these products is essential for their use in resource-limited settings.
- Cost Reduction: The cost of synthetic biology diagnostics can be a barrier to their adoption, particularly in low-income countries. Reducing the cost of these diagnostics through process optimization and economies of scale is essential to improve access.
- User Training and Acceptance: Healthcare providers and patients need to be trained on how to use synthetic biology diagnostics correctly. Ensuring user acceptance and compliance is crucial for the successful implementation of these technologies.
- Data Connectivity and Integration: Integrating synthetic biology-based POCD into electronic health records and surveillance systems can be challenging. Developing robust data connectivity and integration solutions is essential to maximize the impact of these diagnostics.
- Ethical Considerations: The use of synthetic biology in diagnostics raises ethical considerations related to privacy, data security, and informed consent. Addressing these ethical issues is crucial to ensure responsible development and deployment of these technologies.
5.2. How Can These Obstacles Be Overcome?
Overcoming the obstacles to widespread adoption of synthetic biology diagnostics requires a multi-faceted approach involving collaboration between researchers, industry, regulatory agencies, and healthcare providers. Key strategies include:
- Streamlining Regulatory Pathways: Regulatory agencies should develop clear and streamlined pathways for the approval of synthetic biology diagnostics, reducing the time and cost associated with regulatory compliance.
- Investing in Manufacturing Infrastructure: Governments and industry should invest in the development of robust and scalable manufacturing infrastructure for synthetic biology diagnostics.
- Improving Stability and Shelf Life: Researchers should focus on developing strategies to improve the stability and shelf life of synthetic biology-based reagents and devices, such as lyophilization, encapsulation, and storage optimization.
- Reducing Costs Through Innovation: Researchers and industry should explore innovative approaches to reduce the cost of synthetic biology diagnostics, such as using low-cost materials, optimizing manufacturing processes, and developing multiplexed assays.
- Developing User-Friendly Interfaces: Synthetic biology diagnostics should be designed with user-friendly interfaces that are easy to use and interpret, even by individuals with limited training.
- Integrating Data Connectivity Solutions: Developers should integrate data connectivity solutions into synthetic biology diagnostics, allowing for seamless integration with electronic health records and surveillance systems.
- Addressing Ethical Concerns: Stakeholders should engage in open and transparent discussions about the ethical considerations raised by synthetic biology diagnostics and develop guidelines to ensure responsible development and deployment.
5.3. What Are the Future Directions for Synthetic Biology-Based Point-of-Care Diagnostics?
The future of synthetic biology-based point-of-care diagnostics (POCD) is promising, with several exciting directions for innovation and development. A 2023 forecast in “Trends in Biotechnology” outlines potential advancements:
- Multiplexed Diagnostics: Developing multiplexed POCD that can simultaneously detect multiple pathogens or biomarkers will improve diagnostic efficiency and reduce costs.
- Personalized Diagnostics: Tailoring POCD to individual patients based on their genetic background, immune status, and other factors will enable personalized treatment strategies and improved outcomes.
- Wearable and Implantable Diagnostics: Developing wearable and implantable diagnostic devices that can continuously monitor biomarkers of infection will provide real-time feedback and enable early detection of disease.
- Artificial Intelligence Integration: Integrating artificial intelligence (AI) with synthetic biology-based POCD will enable automated data analysis, improved diagnostic accuracy, and personalized treatment recommendations.
- Synthetic Biology-Based Therapeutics: Combining synthetic biology-based diagnostics with synthetic biology-based therapeutics will enable closed-loop systems that can automatically detect and treat infections.
- Global Health Applications: Expanding the use of synthetic biology-based POCD in global health settings will improve access to diagnostic testing and reduce the burden of infectious diseases in resource-limited countries.
- Biosecurity Applications: Developing synthetic biology-based POCD for biosecurity applications will enable rapid detection of biothreat agents and improve preparedness for bioterrorism events.
Synthetic biology-based point-of-care diagnostics are transforming infectious disease detection, offering rapid, accessible, and precise tools. These innovations enhance disease management and patient outcomes, particularly in resource-limited settings.
For those seeking reliable automotive information, CAR-TOOL.EDU.VN offers detailed resources to enhance your knowledge and skills.
Need expert advice? Contact us at 456 Elm Street, Dallas, TX 75201, United States, or via Whatsapp at +1 (641) 206-8880. Explore our website CAR-TOOL.EDU.VN for more information.
FAQ: Synthetic Biology-Based Point-of-Care Diagnostics for Infectious Disease
Q1: What are synthetic biology-based point-of-care diagnostics?
Synthetic biology-based point-of-care diagnostics are diagnostic tests that utilize synthetic biology principles to create rapid, portable, and user-friendly devices for detecting infectious diseases at the point of care, eliminating the need for centralized laboratory testing. These diagnostics often involve engineered biological systems that can precisely detect and amplify signals from target pathogens, providing results in minutes.
Q2: How do synthetic biology diagnostics differ from traditional diagnostic methods?
Synthetic biology diagnostics differ from traditional methods by offering enhanced specificity, sensitivity, and speed. Traditional methods often require complex laboratory procedures and specialized equipment, while synthetic biology diagnostics are designed for rapid, on-site testing with minimal training. Synthetic biology enables the creation of highly specific recognition elements and amplification mechanisms, improving diagnostic accuracy.
Q3: What types of infectious diseases can be diagnosed using these diagnostics?
Synthetic biology-based point-of-care diagnostics can be used to diagnose a wide range of infectious diseases, including respiratory infections (e.g., influenza, COVID-19), sexually transmitted infections (e.g., HIV, chlamydia), gastrointestinal infections, bloodborne infections (e.g., malaria, dengue fever), and healthcare-associated infections (e.g., MRSA). These diagnostics are versatile and can be adapted to detect emerging pathogens.
Q4: What are the advantages of using point-of-care diagnostics in resource-limited settings?
In resource-limited settings, point-of-care diagnostics offer improved accessibility, rapid results, ease of use, and portability. They reduce the need for centralized laboratory testing, lower healthcare costs, and enable timely clinical decisions, leading to better patient outcomes and enhanced disease surveillance.
Q5: How can synthetic biology diagnostics help in combating pandemics?
Synthetic biology diagnostics can play a crucial role in combating pandemics by enabling rapid and widespread detection of infectious agents. They facilitate rapid detection, widespread screening, decentralized testing, and continuous surveillance of viral strains, helping to identify infected individuals and implement control measures effectively.
Q6: What are the main challenges in the widespread adoption of synthetic biology diagnostics?
The main challenges include regulatory hurdles, scalability and manufacturing issues, limited stability and shelf life, high costs, the need for user training, and ethical considerations. Overcoming these obstacles requires collaboration between researchers, industry, regulatory agencies, and healthcare providers.
Q7: How are researchers addressing the challenges of stability and shelf life in these diagnostics?
Researchers are addressing stability and shelf life challenges through strategies such as lyophilization, encapsulation, and storage optimization. These methods aim to preserve the integrity of biological reagents and devices under harsh environmental conditions, ensuring their effectiveness in various settings.
Q8: What role does artificial intelligence play in the future of synthetic biology diagnostics?
Artificial intelligence (AI) can enhance synthetic biology diagnostics by enabling automated data analysis, improving diagnostic accuracy, and providing personalized treatment recommendations. AI algorithms can process complex diagnostic data, identify patterns, and assist healthcare providers in making informed decisions.
Q9: Are there any ethical concerns associated with the use of synthetic biology in diagnostics?
Yes, ethical concerns include privacy, data security, and informed consent. It is crucial to address these ethical issues to ensure responsible development and deployment of synthetic biology diagnostics. Stakeholders should engage in open and transparent discussions to develop guidelines that protect patient rights and promote trust.
Q10: How can I learn more about synthetic biology and point-of-care diagnostics?
You can learn more about synthetic biology and point-of-care diagnostics through academic journals, scientific conferences, and online resources such as CAR-TOOL.EDU.VN. Professional organizations and research institutions also offer educational programs and training opportunities in this field.
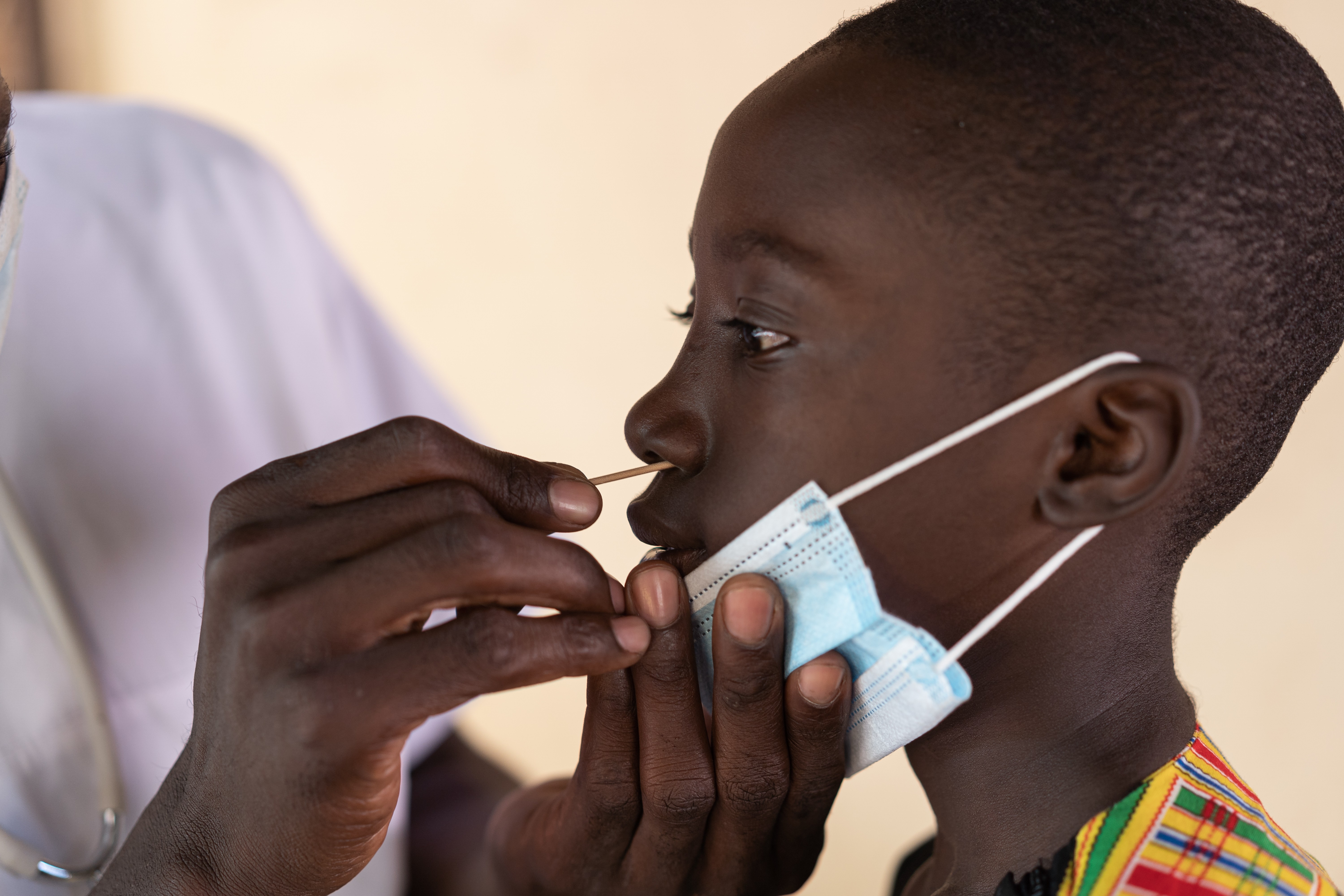 Technician using diagnostic toolSynthetic biology diagnostic tools offer accessible point-of-need solutions, facilitating early disease detection and treatment.
Technician using diagnostic toolSynthetic biology diagnostic tools offer accessible point-of-need solutions, facilitating early disease detection and treatment.
