Point-of-care infectious disease diagnostics are rapid, on-site tests that provide crucial information for timely treatment and disease control, as emphasized by CAR-TOOL.EDU.VN. These diagnostics play a vital role in personal “radar” in healthcare settings. The rise of technologies like microfluidics and plasmonics enhance these tests, offering accurate, accessible solutions for identifying pathogens and guiding effective interventions.
Contents
- 1. What Are Point Of Care Infectious Disease Diagnostics (POC IDD)?
- 2. Why Is Point-Of-Care Testing Important for Infectious Diseases?
- 3. What Are the Different Types of Biomarkers Used in Point-Of-Care Tests for Infectious Diseases?
- 4. How Do Nucleic Acid Tests (NATs) Work in Point-Of-Care Settings?
- 5. What Role Do Antibodies Play in Point-Of-Care Diagnostics for Infectious Diseases?
- 6. How Are Pathogen Proteins Used as Biomarkers in Point-Of-Care Tests?
- 7. What Are Circulating MicroRNAs, and How Are They Used in Point-Of-Care Diagnostics?
- 8. What Are Microfluidic Technologies, and How Are They Used in Point-Of-Care Diagnostics?
- 9. How Is Microfluidics Used in Malaria Diagnosis at the Point of Care?
- 10. What Are the Advancements in Microfluidic Devices for Tuberculosis (TB) Diagnosis?
- 11. How Are Microfluidic Chips Used for HIV Detection and Monitoring?
- 12. How Are Paper-Based Microfluidic Devices Used in Point-Of-Care Diagnostics?
- 13. What Are Plasmonic Technologies, and How Do They Enhance Point-Of-Care Diagnostics?
- 14. How Does Localized Surface Plasmon Resonance (LSPR) Enhance Point-Of-Care Testing?
- 15. What Is Surface-Enhanced Raman Scattering (SERS), and How Is It Used in Point-Of-Care Diagnostics?
- 16. What Are Some Examples of Point-Of-Care Tests for Dengue Virus?
- 17. How Are Point-Of-Care Diagnostics Used in the Detection of the Ebola Virus?
- 18. What Is the Role of Point-Of-Care Testing in Managing HIV/AIDS in Resource-Limited Settings?
- 19. How Are Point-Of-Care Tests Improving Cervical Cancer Screening in Developing Countries?
- 20. How Is Point-Of-Care Diagnostics Being Used to Combat the Zika Virus?
- 21. What Are the Challenges and Future Directions for Point-Of-Care Infectious Disease Diagnostics?
- Frequently Asked Questions (FAQs)
1. What Are Point Of Care Infectious Disease Diagnostics (POC IDD)?
Point Of Care Infectious Disease Diagnostics (POC IDD) are tests performed near the patient that provide quick results, enabling immediate medical decisions. According to the World Health Organization (WHO), POC IDD should be affordable, sensitive, specific, user-friendly, rapid, robust, equipment-free, and deliverable. This approach helps prevent disease transmission and ensures timely treatment, particularly in resource-limited settings.
These diagnostics offer several key advantages:
- Rapid Results: Enable quick clinical decisions and prompt treatment.
- Accessibility: Suitable for remote and resource-limited areas.
- Ease of Use: Require minimal training, making them accessible to various healthcare providers.
- Cost-Effectiveness: Reduce the need for expensive lab equipment and specialized personnel.
Early diagnosis of diseases like HIV, malaria, and tuberculosis (TB) prevents transmission and allows for timely interventions, thereby improving patient outcomes and public health.
2. Why Is Point-Of-Care Testing Important for Infectious Diseases?
Point-of-care testing (POCT) is critical because infectious diseases can spread rapidly, necessitating immediate diagnosis and treatment. Studies from the University of California, San Francisco (UCSF), published in “The Lancet” in 2023, show that POCT significantly reduces the time to diagnosis and treatment initiation, leading to better patient outcomes. POCT allows healthcare providers to quickly identify infections, start appropriate therapies, and implement infection control measures.
Key benefits of POCT include:
- Faster Diagnosis: Reducing the time from symptom onset to diagnosis, enabling quicker treatment.
- Improved Patient Outcomes: Early treatment initiation reduces disease severity and transmission.
- Enhanced Infection Control: Rapid identification aids in isolating infected individuals and preventing outbreaks.
- Accessibility in Remote Areas: POCT devices can be used in areas with limited lab infrastructure.
For example, during the Ebola outbreak in West Africa, rapid POCT helped identify and isolate cases more efficiently than traditional lab-based methods, preventing further spread of the disease.
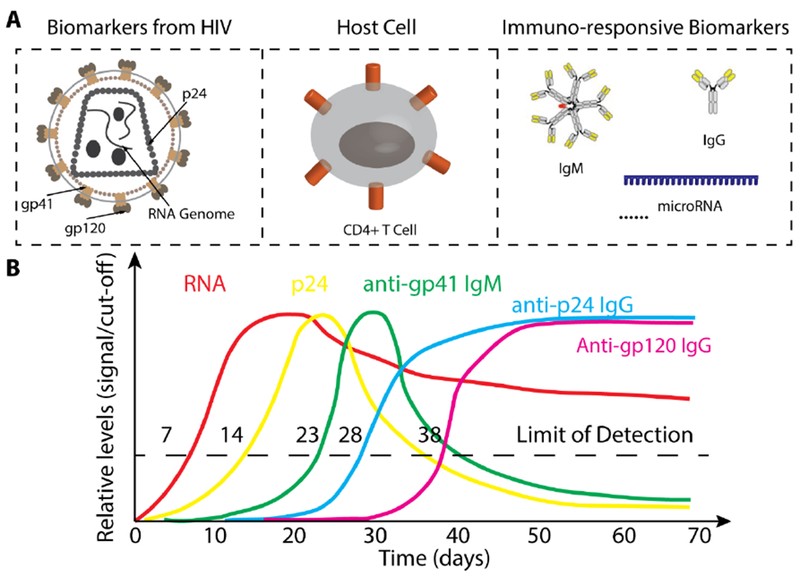
3. What Are the Different Types of Biomarkers Used in Point-Of-Care Tests for Infectious Diseases?
Point-of-care tests (POCT) for infectious diseases utilize various biomarkers to detect pathogens quickly and accurately. Biomarkers include nucleic acids (RNA or DNA), antibodies, pathogen proteins, and circulating microRNAs. Nucleic acid tests (NATs) detect pathogen-specific sequences, while antibody tests identify the presence of anti-pathogen antibodies.
- Nucleic Acids: Pathogen RNA or DNA, providing direct evidence of infection.
- Antibodies: Indicate the body’s immune response to a pathogen.
- Pathogen Proteins: Specific proteins produced by the pathogen, such as HIV’s p24 protein.
- Circulating microRNAs: Small RNA molecules that reflect the host’s immune response.
Each biomarker provides unique information about the infection stage and can be used to guide treatment decisions.
 Pathogen Nucleic Acids
Pathogen Nucleic Acids
4. How Do Nucleic Acid Tests (NATs) Work in Point-Of-Care Settings?
Nucleic Acid Tests (NATs) detect pathogen-specific DNA or RNA, providing direct evidence of infection. In point-of-care (POC) settings, NATs need to be rapid, simple, and cost-effective. According to research from Harvard Medical School published in “Nature Biotechnology” in 2022, isothermal amplification methods like Recombinase Polymerase Amplification (RPA) and Loop-Mediated Isothermal Amplification (LAMP) are replacing traditional PCR due to their simplicity and speed.
- Isothermal Amplification: These methods amplify nucleic acids at a constant temperature, eliminating the need for a thermal cycler.
- Microfluidic Integration: NATs are integrated into microfluidic devices to automate sample preparation and analysis.
- Simplified Procedures: POC NATs require minimal sample preparation, reducing the risk of contamination and user error.
These advancements make NATs more accessible in resource-limited settings, improving diagnostic capabilities and patient care.
5. What Role Do Antibodies Play in Point-Of-Care Diagnostics for Infectious Diseases?
Antibodies play a vital role in point-of-care diagnostics (POC) as biomarkers for evaluating the infectious state. During infection, the immune system produces antibodies, which can be detected to diagnose diseases like HIV and dengue. Antibody tests are often easier to develop than antigen tests and can detect antibodies even when pathogen levels are low.
- Immunoassays: These tests detect antibodies in blood or other bodily fluids.
- Lateral Flow Assays: Rapid tests like the HIV antibody test provide results in minutes.
- Disease Diagnosis: Useful in late-stage infections when pathogen levels decrease.
However, antibody tests may not be suitable for early-stage detection or in infants with maternal antibodies, highlighting the need for complementary diagnostic approaches.
6. How Are Pathogen Proteins Used as Biomarkers in Point-Of-Care Tests?
Pathogen proteins, such as capsid or envelope proteins, serve as valuable biomarkers in point-of-care tests (POCT). For instance, the HIV virus capsid protein p24 can be detected early in the infection process, even before antibodies are detectable. The Alere Determine™ HIV-1/2 Ag/Ab Combo rapid test detects both HIV antibodies and the p24 antigen.
- Early Detection: p24 can be detected before seroconversion.
- Rapid Testing: Enables quick results for timely treatment decisions.
- Complementary to Antibody Tests: Useful when antibody tests are not suitable.
Unlike nucleic acids, proteins are not easily amplified, requiring highly sensitive detection methods for effective POCT applications.
7. What Are Circulating MicroRNAs, and How Are They Used in Point-Of-Care Diagnostics?
Circulating microRNAs (miRNAs) are small, non-coding RNA molecules that regulate gene expression and play critical roles in the host immune response. These miRNAs are released into extracellular environments and are stable in body fluids, making them potential biomarkers for monitoring pathological states. Research published in “PLoS One” in 2021 by researchers at Johns Hopkins University demonstrates that specific miRNA expression signatures can be used to diagnose TB and HIV.
- Gene Regulation: miRNAs regulate gene expression post-transcriptionally.
- Immune Response: Play a role in the host’s immune response during infection.
- Stability: Stable in body fluids, making them reliable biomarkers.
Circulating miRNA expression signatures can potentially monitor and diagnose various infectious diseases, providing valuable insights into disease progression and treatment response.
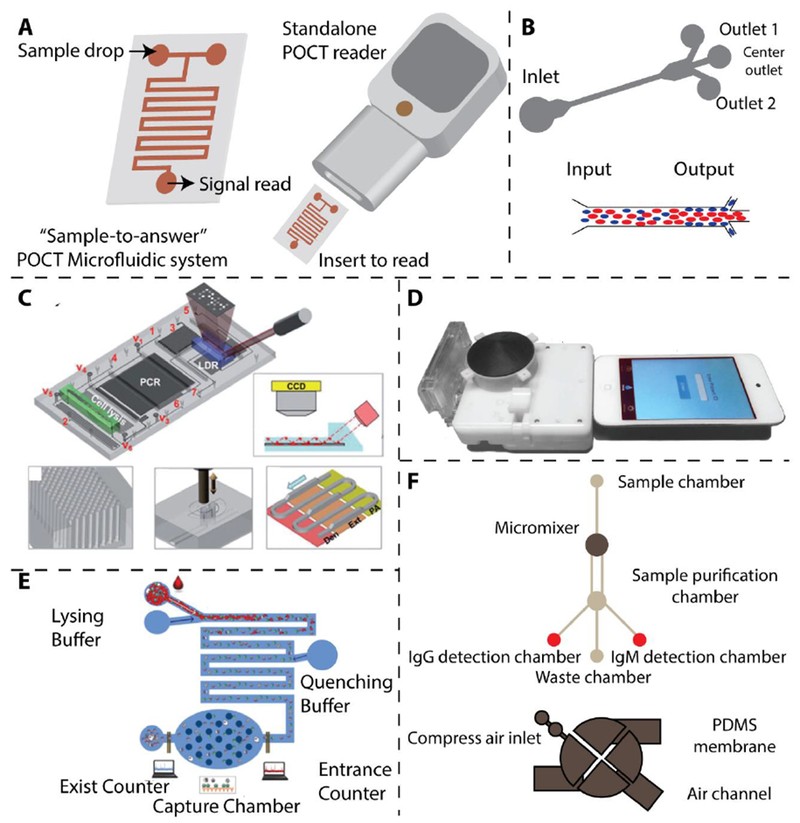
8. What Are Microfluidic Technologies, and How Are They Used in Point-Of-Care Diagnostics?
Microfluidic technologies manipulate small volumes of fluids (10−9 to 10−18 L), offering precise control over fluid transport, mixing, and reactions. These technologies are ideal for point-of-care (POC) testing due to their automation, integration, and miniaturization capabilities. A sample-to-answer microfluidic system integrates all diagnostic steps into a portable chip.
- Automation: Automates sample preparation, reaction, and detection.
- Integration: Integrates multiple diagnostic steps into a single device.
- Miniaturization: Creates compact and portable devices.
Microfluidic devices have been developed for detecting malaria, tuberculosis, HIV, and dengue, offering rapid and accurate results in resource-limited settings.
 Microfluidics
Microfluidics
9. How Is Microfluidics Used in Malaria Diagnosis at the Point of Care?
Microfluidics can be used in malaria diagnosis by leveraging the deformability of infected red blood cells (iRBCs). As malaria parasites mature, iRBCs lose deformability. Microfluidic devices can separate iRBCs based on this property, concentrating them for detection.
- Deformability-Based Separation: iRBCs are separated based on their reduced deformability.
- High Isolation Rate: Devices can isolate over 80% of iRBCs in trophozoite/schizont stages.
This approach allows for rapid and efficient malaria diagnosis, especially in areas where traditional microscopy is not feasible.
10. What Are the Advancements in Microfluidic Devices for Tuberculosis (TB) Diagnosis?
Advancements in microfluidic devices for TB diagnosis include automated systems that integrate cell lysis, DNA isolation, PCR amplification, and signal readout into a single cartridge. These devices can detect single-base variations in multi-drug resistant forms of M. tuberculosis.
- Integrated System: Automates multiple diagnostic steps.
- Single-Base Variation Detection: Identifies drug-resistant strains.
- Micropillar Arrays: Increase the interaction surface for DNA adsorption and signal enhancement.
These devices provide rapid and accurate TB diagnosis with minimal technical expertise, making them valuable in TB-endemic regions.
11. How Are Microfluidic Chips Used for HIV Detection and Monitoring?
Microfluidic chips are used for HIV detection and monitoring by integrating immunoassays and electrical impedance measurements. For instance, microfluidic chips can simultaneously detect HIV and syphilis using silver-enhanced immunoassays.
- Silver-Enhanced Immunoassays: Increase the sensitivity and specificity of ELISA-like assays.
- Mobile Device Integration: Microfluidic devices can be integrated into small cartridges for easy readout by mobile devices.
- Electrical Impedance Measurement: Used to count CD4+ and CD8+ T cells for HIV monitoring.
These advancements enable rapid and accurate HIV diagnosis and monitoring in resource-limited settings, aligning with the WHO’s recommendations.
12. How Are Paper-Based Microfluidic Devices Used in Point-Of-Care Diagnostics?
Paper-based microfluidic devices offer a low-cost and user-friendly solution for infectious disease detection in point-of-care (POC) settings. These devices use paper as a substrate for fluid transport and reaction, making them portable and disposable.
- Low Cost: Reduces the overall cost of diagnostic testing.
- User-Friendly: Simple to use with minimal training required.
- Colorimetric Readout: Allows for easy visual detection of results.
Paper-based microfluidic devices have been developed for detecting HIV antibodies, Ebola RNA, TB-DNA, and Zika RNA, making them valuable tools in resource-limited circumstances.
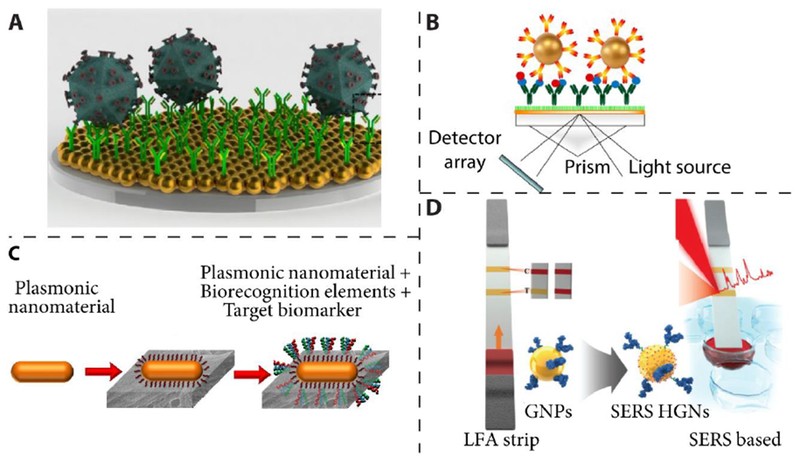
13. What Are Plasmonic Technologies, and How Do They Enhance Point-Of-Care Diagnostics?
Plasmonic technologies study the interaction between light and the conductive electrons of metallic nanomaterials, such as gold and silver. These technologies enhance point-of-care diagnostics by providing label-free detection, facile optical tunability, and high sensitivity to the surrounding medium.
- Label-Free Detection: Eliminates the need for fluorescent or enzymatic labels.
- Optical Tunability: Allows for precise control over the interaction between light and nanomaterials.
- High Sensitivity: Detects low concentrations of biomarkers.
Plasmonic nanomaterials, such as gold nanoparticles, are used in various optical sensing platforms, including localized surface plasmon resonance (LSPR) and surface-enhanced Raman scattering (SERS) sensors.
14. How Does Localized Surface Plasmon Resonance (LSPR) Enhance Point-Of-Care Testing?
Localized Surface Plasmon Resonance (LSPR) enhances point-of-care testing by providing high sensitivity to refractive index changes. This technique allows for label-free and fluorescence-free detection of biomarkers, such as HIV viral load in whole blood.
- Refractive Index Sensitivity: LSPR is highly sensitive to changes in the surrounding medium’s refractive index.
- Label-Free Detection: Eliminates the need for labels, simplifying the assay.
- Repeatable Detection: Allows for repeated measurements, enhancing reliability.
LSPR can detect and quantify multiple HIV subtypes with high sensitivity, specificity, and relatively short assay times, making it valuable in point-of-care diagnostics.
 Localized Surface Plasmon Resonance
Localized Surface Plasmon Resonance
15. What Is Surface-Enhanced Raman Scattering (SERS), and How Is It Used in Point-Of-Care Diagnostics?
Surface-Enhanced Raman Scattering (SERS) involves the amplification of Raman scattering from analytes adsorbed on or nearby a nanostructured metal surface. This technique offers ultrahigh sensitivity and is used in point-of-care diagnostics for detecting various biomarkers.
- Signal Amplification: SERS provides large signal amplification, enhancing detection sensitivity.
- Uniform Enhancement: Efforts are dedicated to designing SERS devices with uniform signal enhancement.
- Lateral Flow Assays: SERS-based lateral flow assays have been developed for detecting staphylococcal enterotoxin B with ultrahigh sensitivity.
SERS-based assays are valuable for rapid and sensitive detection of infectious disease biomarkers, improving diagnostic capabilities in point-of-care settings.
16. What Are Some Examples of Point-Of-Care Tests for Dengue Virus?
Point-of-care tests (POCTs) for Dengue virus (DENV) include rapid diagnostic tests (RDTs) that detect nonstructural protein 1 (NS1) antigen and DENV-specific antibodies (IgM and IgG). These tests provide quick, on-site confirmation of suspected cases, crucial for timely management of severe dengue diseases.
- NS1 Antigen Detection: Detects the DENV NS1 protein, indicating active infection.
- Antibody Detection: Identifies IgM and IgG antibodies, indicating recent or past infection.
- Integrated Microfluidic Devices: Detect specific IgG and IgM antibodies using magnetic microbeads and micromixers.
These POCTs are essential for avoiding overtreatment of cases with similar symptoms but no DENV infection, especially in resource-limited regions.
17. How Are Point-Of-Care Diagnostics Used in the Detection of the Ebola Virus?
Point-of-care diagnostics are crucial during Ebola virus (EBOV) outbreaks due to the virus’s highly contagious nature and rapid disease progression. Rapid diagnostic tests (RDTs) like the ReEBOV Antigen Rapid Test kit provide quick results, enabling rapid isolation and treatment.
- Antigen Detection: RDTs detect EBOV antigens, providing rapid confirmation of infection.
- Lateral Flow Assays: Detect Sudan virus with customized smartphone applications for data collection.
- Surface-Enhanced Raman Spectroscopy (SERS): Differentiates Ebola from other febrile diseases.
These POC tools are essential for containing outbreaks, especially in areas with limited laboratory resources.
18. What Is the Role of Point-Of-Care Testing in Managing HIV/AIDS in Resource-Limited Settings?
Point-of-care (POC) testing is essential for managing HIV/AIDS in resource-limited settings, where access to central clinical laboratories is limited. POC devices can accurately detect and monitor HIV/AIDS, enabling timely treatment and prevention.
- Early Detection: POC tests can detect HIV infection early, preventing unknowing transmissions.
- Therapy Monitoring: Enumeration of CD4+ T-lymphocytes and quantitation of HIV viral load can monitor HIV infection.
- Affordable and Easy to Use: POC devices should be inexpensive, easy to use, and disposable to enable widespread use.
WHO emphasizes the urgent demand for POC devices in resource-limited settings, emphasizing their role in improving HIV/AIDS management.
19. How Are Point-Of-Care Tests Improving Cervical Cancer Screening in Developing Countries?
Point-of-care tests (POCTs) are improving cervical cancer screening in developing countries by providing simple, affordable, and accurate alternatives to traditional Pap-based screening. HPV DNA testing at the point of care can significantly reduce cervical cancer incidence and mortality.
- HPV DNA Testing: Detects oncogenic types of HPV, the primary cause of cervical cancer.
- Visual Inspection with Acetic Acid (VIA): An alternative test recommended by WHO for resource-limited areas.
- High Sensitivity and Specificity: POCT platforms for HPV virus improve cervical cancer prevention.
These POCTs address the limitations of expensive laboratory settings and unreliable recall systems, making screening more accessible.
20. How Is Point-Of-Care Diagnostics Being Used to Combat the Zika Virus?
Point-of-care diagnostics (POC) are crucial for combating Zika virus (ZIKV) due to the virus’s potential to cause congenital microcephaly and other severe neurological defects. Accurate and rapid detection of ZIKV is critical for proper and timely therapeutic interventions.
- Infection Spread Tracking: POC tests play a vital role in tracking infection spread and managing risk during pregnancy.
- Treatment and Vaccine Monitoring: Used for monitoring treatment and vaccine efficacy.
- Blood Supply Safety: Ensures the safety of blood supplies.
Simple, accurate, and rapid POC diagnostic tools for ZIKV detection are key to effective treatment and prevention, particularly in regions where ZIKV is prevalent.
21. What Are the Challenges and Future Directions for Point-Of-Care Infectious Disease Diagnostics?
Despite significant advancements, challenges remain in point-of-care infectious disease diagnostics. These include the need for biomarkers with better sensitivity and specificity, multiplex functionality to detect multiple pathogens, and stringent clinical validations.
- Improved Biomarkers: Identifying and characterizing more accurate biomarkers.
- Multiplex Testing: Developing tests that can detect multiple pathogens simultaneously.
- Clinical Validation: Conducting thorough clinical validations to ensure reliability.
Future directions include integrating microfluidics and plasmonics, addressing practical issues such as infection control and information technology connectivity, and optimizing clinical pathways for successful implementation.
Finding the right tools and information is crucial for efficiently managing automotive repairs. At CAR-TOOL.EDU.VN, we understand the challenges you face in finding reliable parts and tools. Our website offers detailed information on a wide range of auto parts and repair tools, complete with specifications, comparisons, and user reviews. We can help you locate the right parts, compare costs and features, and connect with trusted suppliers, saving you time and ensuring quality.
Ready to simplify your search for auto parts and repair tools? Contact us today for expert guidance and tailored solutions. Reach out via:
- Address: 456 Elm Street, Dallas, TX 75201, United States
- WhatsApp: +1 (641) 206-8880
- Website: CAR-TOOL.EDU.VN
Let CAR-TOOL.EDU.VN be your trusted partner in automotive repairs, providing you with the information and support you need to succeed.
Frequently Asked Questions (FAQs)
Q1: What types of infectious diseases can be diagnosed using point-of-care tests?
A1: Point-of-care tests can diagnose a wide range of infectious diseases, including HIV, malaria, tuberculosis, dengue, Ebola, Zika, and HPV.
Q2: How long does it take to get results from a point-of-care infectious disease diagnostic test?
A2: Results from point-of-care tests can be available in as little as a few minutes to an hour, depending on the type of test.
Q3: Are point-of-care tests as accurate as lab-based tests?
A3: Point-of-care tests are designed to be highly accurate, though their sensitivity and specificity can vary. They are often used for rapid screening and may be followed by lab-based tests for confirmation.
Q4: Can point-of-care tests be used at home?
A4: Some point-of-care tests, such as HIV self-tests, are designed for home use, but most are intended for use by healthcare professionals in clinical settings.
Q5: What are the benefits of using microfluidic devices in point-of-care diagnostics?
A5: Microfluidic devices offer automation, integration, and miniaturization, enabling rapid and precise diagnostics with minimal sample volume.
Q6: How do plasmonic technologies enhance point-of-care diagnostics?
A6: Plasmonic technologies provide label-free detection, optical tunability, and high sensitivity, allowing for the detection of low concentrations of biomarkers.
Q7: What is the role of circulating microRNAs in infectious disease diagnostics?
A7: Circulating microRNAs are stable in body fluids and can serve as biomarkers for monitoring pathological states and immune responses during infection.
Q8: What are the challenges in developing point-of-care tests for infectious diseases?
A8: Challenges include the need for improved biomarkers, multiplex functionality, stringent clinical validation, and addressing practical issues like infection control and IT connectivity.
Q9: How can point-of-care testing improve patient outcomes?
A9: Point-of-care testing enables faster diagnosis, quicker treatment initiation, and enhanced infection control, leading to improved patient outcomes and reduced disease transmission.
Q10: Where can I find reliable auto parts and repair tools?
A10: CAR-TOOL.EDU.VN offers detailed information, specifications, comparisons, and user reviews on a wide range of auto parts and repair tools, helping you make informed decisions.
