Point-of-care Diagnostics: Recent Developments In A Connected Age are revolutionizing healthcare by providing rapid, on-site testing and immediate results, enhancing patient care and streamlining clinical workflows. At CAR-TOOL.EDU.VN, we understand the importance of staying informed about these advancements in diagnostic solutions, ensuring you have access to the latest tools and information. This helps in improving efficiency and accuracy in automotive repairs.
Contents
- 1. Understanding Point-Of-Care Diagnostics
- 2. Key Technological Components of Point-Of-Care Diagnostics
- 2.1. Assay Chemistry: Affinity Reagents and Materials
- 2.2. Microfluidics: Assay Integration
- 2.3. Connected Instrumentation
- 2.4. Data Analytics
- 2.5. Systems Integration
- 3. Clinical and Non-Technological Components
- 3.1. Clinical Workflow
- 3.2. Regulatory Guidance
- 3.3. Reimbursement
- 3.4. Legislation
- 4. Use-Case Scenarios for Point-Of-Care Diagnostics
- 4.1. Use Case 1: Clinic Level with Moderate Budget
- 4.2. Use Case 2: In the Field with Moderate Budget
- 4.3. Use Case 3: Clinic Level with Constrained Budget
- 4.4. Use Case 4: In the Field with Constrained Budget
- 5. Recent Advances and Future Trends
- 5.1. Integration of Technologies
- 5.2. Wearable Sensors
- 5.3. Molecular Diagnostics
- 5.4. Telehealth
- 6. Conclusion: Transforming Healthcare with Point-Of-Care Diagnostics
- 7. Frequently Asked Questions (FAQ)
- 7.1. What are the main advantages of point-of-care diagnostics?
- 7.2. How do microfluidics improve point-of-care testing?
- 7.3. What role do smartphones play in point-of-care diagnostics?
- 7.4. What are the main challenges in implementing point-of-care diagnostics?
- 7.5. How is point-of-care diagnostics impacting chronic disease management?
- 7.6. What are some examples of successful point-of-care diagnostic devices?
- 7.7. How do regulatory agencies ensure the safety and accuracy of point-of-care diagnostic devices?
- 7.8. What impact has point-of-care diagnostics had on reducing healthcare costs?
- 7.9. How are paper-based diagnostics being used in resource-limited settings?
- 7.10. What future innovations can we expect in point-of-care diagnostics?
1. Understanding Point-Of-Care Diagnostics
What is point-of-care diagnostics, and why is it important?
Point-of-care diagnostics (POCD) refers to medical diagnostic testing performed near or at the site of patient care, rather than in a centralized laboratory. This approach significantly reduces the time required to obtain results, enabling faster clinical decision-making and improved patient outcomes. The rise of POCD is crucial in today’s connected age, driven by advancements in technology and the increasing need for rapid, accessible healthcare solutions. According to a study by the World Health Organization, POCD can improve the management of infectious diseases in resource-limited settings by up to 30%. This highlights the transformative potential of POCD in various healthcare scenarios.
The benefits of POCD are numerous:
- Faster Results: Immediate results allow for quicker diagnosis and treatment.
- Accessibility: Testing can be performed in remote or underserved areas.
- Efficiency: Streamlines clinical workflows, reducing the burden on central laboratories.
- Patient Convenience: Reduces the need for multiple visits to healthcare facilities.
- Cost-Effective: Can lower overall healthcare costs by reducing hospital stays and unnecessary treatments.
For automotive professionals, understanding POCD principles can translate into better diagnostic approaches for vehicle systems. Just as POCD offers immediate insights into a patient’s condition, advanced automotive diagnostic tools provide real-time data on a vehicle’s performance. Staying updated with these trends can enhance the efficiency and accuracy of your repair services, aligning with the fast-paced demands of the industry.
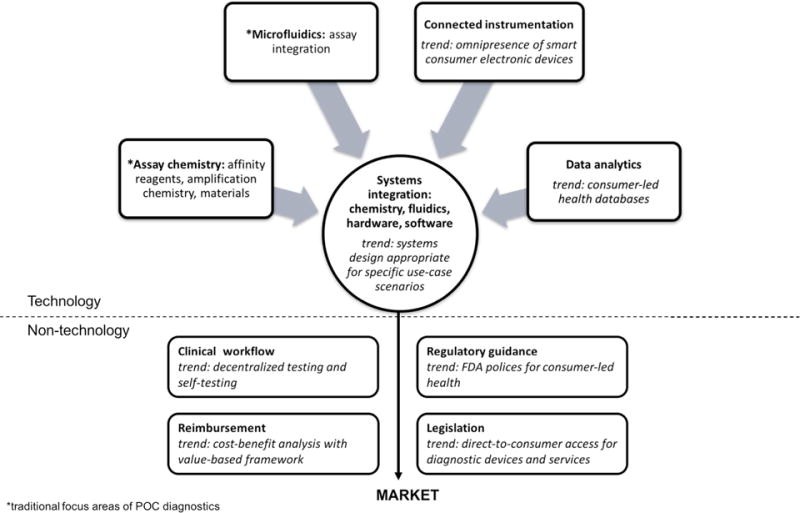
2. Key Technological Components of Point-Of-Care Diagnostics
What are the core technologies driving point-of-care diagnostics?
Several key technological components drive the advancements in point-of-care diagnostics, integrating chemistry, fluidics, hardware, and software to create efficient and user-friendly diagnostic devices. At CAR-TOOL.EDU.VN, we recognize how similar integrated systems have revolutionized automotive diagnostics, providing mechanics with comprehensive data for quick and accurate repairs.
2.1. Assay Chemistry: Affinity Reagents and Materials
How do advancements in assay chemistry improve diagnostic accuracy?
Affinity reagents, such as antibodies and aptamers, play a crucial role in isolating, detecting, and quantifying target molecules in POCD. While antibodies are widely used, aptamers—short, single-stranded oligonucleotides—offer high specificity and affinity target binding. Chemical modifications to aptamers can improve their bioavailability and stability, making them ideal for field-deployed diagnostics. Advances in materials, including nanoparticles (fluorescent, carbon, polymer, silica, and viral), enhance the sensitivity and performance of POCD. For example, strategies involving upconverting phosphor particles can result in signal enhancement without additional amplification steps, simplifying the diagnostic process.
2.2. Microfluidics: Assay Integration
How does microfluidics contribute to integrated point-of-care diagnostic devices?
Microfluidics serves as an integrating force in POCD by seamlessly combining multiple steps of a diagnostic assay, including fluid handling, sample processing, signal amplification, washings, and detection. The development of integrated devices that are self-contained, automated, easy-to-use, and rapid is a key trend in microfluidics. These advancements are particularly beneficial in molecular diagnostics, infectious diseases, chronic diseases, and resource-limited settings. The accessibility of microfluidic technology has also increased, with more opportunities for collaboration and outsourcing to microfluidic foundries and contract research organizations.
2.3. Connected Instrumentation
How do smart devices and connectivity enhance point-of-care diagnostics?
The proliferation of smart consumer electronic devices has significantly impacted POCD, transforming how services are delivered. The key consequences include:
- Ubiquitous Connectivity: Smartphones and tablets enable seamless data transfer and remote monitoring.
- Miniaturization: Allows for the development of smaller, portable diagnostic devices.
- User-Friendly Interfaces: Simplifies operation and data interpretation for healthcare providers and patients.
- Cloud-Based Data Storage: Facilitates data sharing and collaborative analysis.
- Remote Monitoring: Enables continuous monitoring of patients in various healthcare settings.
2.4. Data Analytics
How is data analytics used to improve diagnostic outcomes?
Data analytics plays a crucial role in POCD by leveraging health information collected from various sources (Internet, mobile phones, and social media platforms) for analysis. Consumer-led health data platforms, such as ResearchKit from Apple, link users to studies on various diseases, while search engine queries and website views can be used to infer behaviors associated with medical conditions. Additionally, personalized genomic information, made more accessible by the decreasing cost of next-generation sequencing, aids in analyzing tumor sequencing data and integrating it with clinical outcomes. This integrated view allows researchers to understand a patient’s clinical, diagnostic, and therapeutic outcomes comprehensively.
2.5. Systems Integration
How is it ensured that point-of-care diagnostic devices are user-friendly and effective?
Effective systems integration is crucial for ensuring an appropriate user experience with POCD devices. This involves integrating microfluidics with hardware and software, considering the specific constraints and requirements of different POC settings (emergency room, ambulance, physician’s office, pharmacy, home, or in the field). By recognizing these varying constraints, developers can design POC devices that are user-friendly and effective in diverse healthcare environments.
The automotive industry mirrors this integration, where diagnostic tools combine hardware, software, and data analytics to provide comprehensive vehicle assessments. Just as POCD aims to streamline healthcare, advanced automotive diagnostics seek to improve the efficiency and accuracy of vehicle repairs.
3. Clinical and Non-Technological Components
How do clinical workflows, regulations, and reimbursement models affect point-of-care diagnostics?
Apart from the technological aspects, the effectiveness and adoption of point-of-care diagnostics are significantly influenced by clinical workflows, regulatory guidance, reimbursement models, and legislation.
3.1. Clinical Workflow
How are clinical workflows adapting to point-of-care diagnostics?
As healthcare delivery becomes increasingly decentralized, healthcare professionals and patients are adapting their workflows to incorporate POCD devices. Pathologists and clinicians are adopting POC testing to eliminate transport, processing, and aliquoting processes that take place in core laboratories, creating a more streamlined and faster workflow. This enables more face-to-face interactions between providers and patients to understand test results and plan treatment options. However, ensuring proper oversight and quality assurance over minimally trained or untrained users remains a challenge. Some hospitals and clinics appoint point-of-care coordinators or management teams to ensure consistent procedures, regulatory compliance, correct documentation of results, and end-user assistance.
3.2. Regulatory Guidance
How does regulatory guidance affect point-of-care diagnostic device development?
Regulatory procedures for consumer-targeted health devices and apps are evolving, with the Food and Drug Administration (FDA) regulating products based on their intended functionality and claims. POCD devices that measure biomarker levels are often classified as Class II devices, posing “moderate risk” and requiring 510(k) approval. As POCD devices are increasingly used outside of centralized laboratories, their benefits are weighed against ensuring privacy and security of protected health-related information and legal liability in case of inaccuracies or misuse. Researchers in POCD should be cognizant of the evolving regulations that strive to protect patient health without discouraging technological innovation.
3.3. Reimbursement
What is the future of reimbursement models for point-of-care diagnostic services?
The reimbursement for healthcare services in the U.S. and worldwide is experiencing a structural shift towards a value-based model rather than fee-for-service. In a value-based model, providers are offered incentives (and penalties) depending on their performance based on quality metrics, encouraging continuous patient health measurement and emphasizing prevention and early disease detection. The effectiveness of POCD devices in cost reduction is being evaluated, with some studies showing improvements in clinical operations and cost reductions in ambulatory practices. Consumers and patients are also exhibiting an increasing willingness to pay out of pocket for POC health products and services.
3.4. Legislation
How do laws define the boundaries of direct-to-consumer diagnostic services?
Laws are being passed in the U.S. and worldwide to define the boundaries of direct-to-consumer diagnostic services in response to consumers’ increasing interest in self-management and self-monitoring. Diagnostic test results have become more accessible to patients, with companies like Quest Diagnostics and LabCorp launching patient portals. Legislation defines which genetic tests can be ordered directly by consumers and under which constraints, such as the availability of genetic counseling. Direct-to-consumer access for blood tests and services is also increasing, with corporate wellness programs signing up for enhanced diagnostic services. There continues to be a vigorous discussion over the right balance between consumer access and protection, with federal and state laws continuing to evolve.
Similarly, in the automotive sector, understanding industry standards and regulations is crucial for providing reliable repair services. Just as POCD benefits from clear guidelines and ethical considerations, automotive professionals rely on established protocols and legal frameworks to ensure the safety and quality of their work.
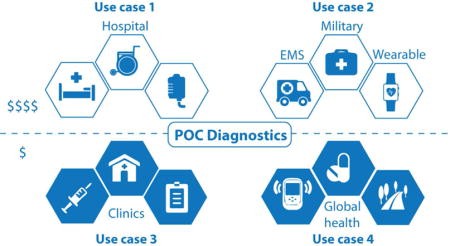
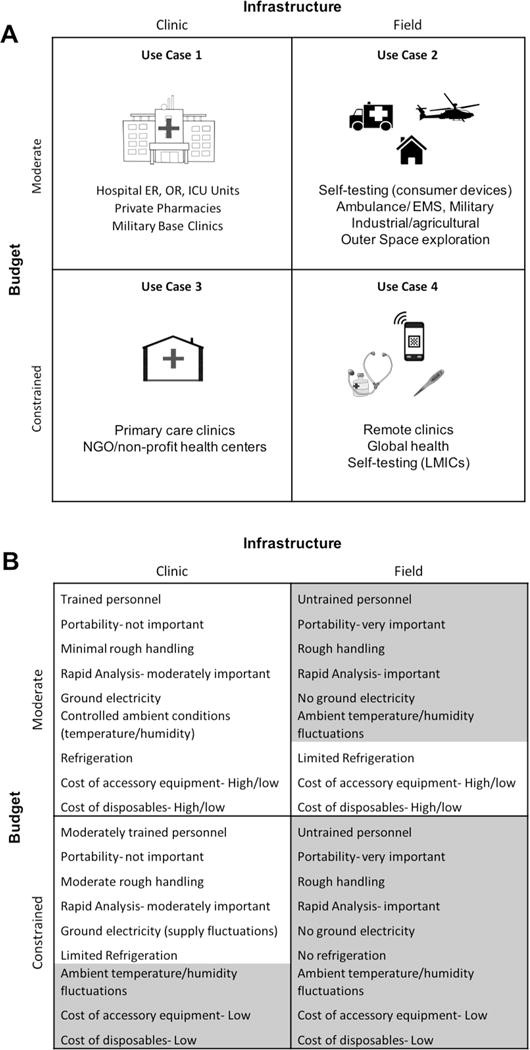
4. Use-Case Scenarios for Point-Of-Care Diagnostics
How can point-of-care diagnostics be applied in various healthcare settings?
Point-of-care diagnostics can be applied in various healthcare settings, each imposing specific design constraints on POCD devices. These settings can be categorized using a 2 × 2 matrix that separates budget (low and moderate) from infrastructure (clinic and home), resulting in four distinct use cases.
4.1. Use Case 1: Clinic Level with Moderate Budget
What are the applications of point-of-care diagnostics in well-equipped clinical settings?
This use case encompasses settings with access to a moderate budget and some level of clinical infrastructure, such as hospital emergency rooms, operating rooms, or intensive care units. In these settings, POCD can be used at the patient’s bedside to provide rapid results that help direct treatment. Moderate throughput in testing of samples is sometimes a desirable feature of the POCD device.
4.2. Use Case 2: In the Field with Moderate Budget
How are point-of-care diagnostic devices used in portable and field settings?
Use Case 2 allows for a relatively flexible and moderate budget for the POCD device but is set apart by differences in the infrastructure available to run the test. These settings often lack trained users and accessory laboratory equipment, making a self-contained, portable device vital. POCD devices in this use case are intended for field use and can contain sophisticated technologies to accomplish their analytical functions. Examples include POCD devices used at home, such as consumer electronics or consumer devices, which need to be operable by untrained users and are self-contained in terms of reagents and disposal.
4.3. Use Case 3: Clinic Level with Constrained Budget
How can point-of-care diagnostics be implemented in clinics with limited resources?
Use Case 3 presents settings with access to laboratory facilities and trained personnel, but where resources and budget are more constrained than in Use Case 1. Examples include primary care clinics in developing countries and nonprofit and non-governmental organization health centers. For researchers developing POCD devices for this use case, significant design considerations must be given to cost; in return, full integration (e.g., sample preparation steps), portability, and sample throughput may not be as critical.
4.4. Use Case 4: In the Field with Constrained Budget
What are the challenges and solutions for point-of-care diagnostics in resource-limited field settings?
Use Case 4 settings represent the greatest set of constraints for POCD, with tight budget constraints and limited infrastructure. These settings include lower-level clinics and health posts, mobile health and community health outreach, and self-testing settings. Design choices in cost, portability, and automation must be made to ensure the POCD device is usable in such settings.
Similarly, automotive diagnostics vary greatly depending on the setting. A well-equipped repair shop (Use Case 1) can handle complex diagnostics, while a mobile mechanic (Use Case 2) requires portable tools. Clinics in developing countries (Use Case 3) need cost-effective solutions, and roadside assistance (Use Case 4) demands simple, reliable devices. CAR-TOOL.EDU.VN supports all these scenarios with a range of diagnostic tools.
5. Recent Advances and Future Trends
What are the latest developments and future directions in point-of-care diagnostics?
5.1. Integration of Technologies
How are chemistry, microfluidics, and connectivity being integrated?
The integration of chemistry, microfluidics, hardware, and software is driving the development of POCD devices. Affinity reagents, such as antibodies and aptamers, are being used to isolate and detect target molecules. Microfluidics is serving as an integrating force, seamlessly combining multiple steps of a diagnostic assay. The rise of smart consumer electronic devices is transforming how services are delivered, with ubiquitous connectivity, miniaturization, user-friendly interfaces, and cloud-based data storage.
5.2. Wearable Sensors
What is the role of wearable sensors in continuous health monitoring?
Wearable sensors are becoming increasingly sophisticated, with the development of ultrathin, flexible electronics that can be mounted on the skin. These sensors can continuously monitor a person’s movements, heartbeat, and temperature, providing a detailed record of their physical activity and vital signs. Wearable sensors have potential applications in monitoring health and wellness, studying disease states, improving surgical procedures, and establishing human-machine interfaces.
5.3. Molecular Diagnostics
How are molecular diagnostic tests being miniaturized for field use?
POC molecular diagnostics tests, for detecting DNA and RNA from pathogens, are difficult to miniaturize, especially for resource-limited settings where they must be low-cost and simple-to-use. Multiple research groups are developing paper-based diagnostic tests to perform molecular diagnostic tests. These tests integrate sample preparation, isothermal DNA amplification, and detection steps into a single paper-based device.
5.4. Telehealth
How will point-of-care diagnostics support advances in telehealth?
As telehealth gains traction, POCD plays a vital role by enabling remote patient monitoring and diagnostics. Connected devices allow healthcare providers to access real-time patient data, facilitating timely interventions and personalized care. This synergy between POCD and telehealth enhances healthcare accessibility, particularly for remote or underserved populations.
The automotive industry is also seeing advances with remote diagnostics. Just as POCD supports telehealth, remote vehicle diagnostics allow mechanics to assess vehicle health from a distance, improving service efficiency and customer convenience.
6. Conclusion: Transforming Healthcare with Point-Of-Care Diagnostics
How will point-of-care diagnostics shape the future of healthcare?
Point-of-care diagnostics is revolutionizing healthcare by providing rapid, accessible, and efficient diagnostic solutions. The integration of chemistry, microfluidics, hardware, and software is driving the development of POCD devices, while clinical workflows, regulatory guidance, reimbursement models, and legislation are shaping their adoption. With ongoing advancements in technology and increasing demand for decentralized healthcare, POCD is poised to transform healthcare delivery, improving patient outcomes and reducing healthcare costs. At CAR-TOOL.EDU.VN, we recognize the importance of staying informed about these advancements, providing you with the knowledge and tools needed to excel in today’s rapidly evolving diagnostic landscape.
7. Frequently Asked Questions (FAQ)
7.1. What are the main advantages of point-of-care diagnostics?
What are the benefits of using point-of-care diagnostics?
The main advantages include faster results, increased accessibility, improved efficiency, greater patient convenience, and cost-effectiveness. POCD enables quicker diagnosis and treatment, reaches remote areas, streamlines clinical workflows, reduces the need for multiple visits, and lowers healthcare costs.
7.2. How do microfluidics improve point-of-care testing?
What role does microfluidics play in point-of-care diagnostics?
Microfluidics integrates multiple diagnostic steps into a single device, including fluid handling, sample processing, signal amplification, and detection. This integration results in self-contained, automated, easy-to-use, and rapid diagnostic devices.
7.3. What role do smartphones play in point-of-care diagnostics?
How are smartphones used in point-of-care diagnostic systems?
Smartphones enhance POCD through ubiquitous connectivity, user-friendly interfaces, and cloud-based data storage. They enable seamless data transfer, simplify operation, and facilitate data sharing and collaborative analysis.
7.4. What are the main challenges in implementing point-of-care diagnostics?
What are the biggest hurdles in adopting point-of-care diagnostic testing?
The main challenges include ensuring proper oversight and quality assurance, navigating evolving regulatory procedures, and addressing reimbursement models. Maintaining data privacy and security and balancing consumer access with protection are also significant concerns.
7.5. How is point-of-care diagnostics impacting chronic disease management?
How does point-of-care diagnostics improve chronic disease care?
POCD enables continuous monitoring of patients with chronic diseases, facilitating timely interventions and personalized care. This leads to better management of conditions such as diabetes, heart disease, and respiratory disorders.
7.6. What are some examples of successful point-of-care diagnostic devices?
What are some of the most effective point-of-care diagnostic devices available?
Examples include blood gas analyzers, wearable sensors, and cartridge-based molecular diagnostic systems for tuberculosis and HIV. These devices have shown success in various clinical and field settings, improving patient outcomes and healthcare efficiency.
7.7. How do regulatory agencies ensure the safety and accuracy of point-of-care diagnostic devices?
How do regulatory bodies guarantee the quality of point-of-care diagnostic tools?
Regulatory agencies like the FDA classify POCD devices based on risk and require appropriate approvals, such as 510(k) for Class II devices. They also monitor privacy, security, and legal liability to ensure patient safety and data protection.
7.8. What impact has point-of-care diagnostics had on reducing healthcare costs?
Does point-of-care diagnostics lead to financial savings in healthcare?
POCD has the potential to reduce healthcare costs by decreasing hospital stays, minimizing unnecessary treatments, and streamlining clinical operations. While some studies show significant cost reductions, others indicate that consumer usage may not always lead to immediate savings.
7.9. How are paper-based diagnostics being used in resource-limited settings?
What role do paper-based diagnostics play in low-resource environments?
Paper-based diagnostics offer low-cost, simple-to-use solutions for resource-limited settings. They are stable, easy to interpret, and require minimal equipment, making them ideal for field use in global health applications.
7.10. What future innovations can we expect in point-of-care diagnostics?
What future developments are anticipated in point-of-care diagnostics?
Future innovations include more sophisticated wearable sensors, improved integration of technologies, and broader applications in telehealth. Advances in molecular diagnostics and the development of new analytical methods will further enhance the capabilities of POCD.
Need detailed information about specific auto parts or repair tools? Want to compare features or find reliable suppliers?
Contact CAR-TOOL.EDU.VN today for expert assistance and personalized recommendations.
Address: 456 Elm Street, Dallas, TX 75201, United States.
Whatsapp: +1 (641) 206-8880.
Website: CAR-TOOL.EDU.VN.
Your success is our priority. Let us help you make informed decisions and achieve excellence in automotive repairs.
 Affinity Reagents
Affinity Reagents
 POC Ecosystem
POC Ecosystem
 POC Use Cases
POC Use Cases