The Inhalable Point-of-care Urinary Diagnostic Platform is an innovative approach for early lung cancer detection, offering rapid, non-invasive results. At CAR-TOOL.EDU.VN, we are dedicated to providing comprehensive insights into cutting-edge diagnostic tools like this, helping you stay informed about the latest advancements in automotive and medical technology. The system provides new hope for accessible and cost-effective diagnostics, including multiplexable paper-based lateral flow assay, protease dysregulation and rapid detection.
Contents
- 1. What is the Inhalable Point-of-Care Urinary Diagnostic Platform?
- 1.1. Key Components of the Platform
- 1.2. How Does It Work?
- 2. What are the Benefits of Using an Inhalable Diagnostic Platform?
- 2.1. Non-Invasive
- 2.2. Rapid Results
- 2.3. Accessibility
- 2.4. Cost-Effectiveness
- 2.5. Early Detection
- 3. How Does the PATROL System Work?
- 3.1. Activity-Based Nanosensors (ABNs)
- 3.2. Inhalation Unit
- 3.3. Lateral Flow Assay (LFA)
- 4. What is the Scientific Evidence Supporting the Platform?
- 4.1. In Vitro Studies
- 4.2. In Vivo Studies
- 4.3. Safety Studies
- 5. What Proteases are Targeted by the ABNs?
- 5.1. Specific Substrates Used
- 6. How Does the Lateral Flow Assay (LFA) Work in Detail?
- 6.1. Components of the LFA Strip
- 6.2. Step-by-Step Process
- 6.3. Multiplexing Capability
- 7. What are the Potential Clinical Applications?
- 7.1. Early Detection of Lung Cancer
- 7.2. Monitoring Treatment Response
- 7.3. Risk Stratification
- 7.4. Screening in Resource-Limited Settings
- 7.5. Personalized Medicine
- 8. What are the Limitations of the Platform?
- 8.1. Sensitivity and Specificity
- 8.2. Standardization
- 8.3. Regulatory Approval
- 8.4. Patient Compliance
- 8.5. Cost and Reimbursement
- 9. What Future Research is Needed?
- 9.1. Clinical Validation
- 9.2. Optimization of ABNs
- 9.3. Expansion of Multiplexing Capability
- 9.4. Integration with Digital Health Technologies
- 9.5. Cost-Effectiveness Analysis
- 10. What are the Implications for the Automotive Industry?
- 10.1. Sensor Technology
- 10.2. Point-of-Care Diagnostics
- 10.3. Materials Science
- 10.4. Air Quality Monitoring
- 10.5. Health and Safety
- FAQ: Inhalable Point-Of-Care Urinary Diagnostic Platform
- 1. What is the Inhalable Point-Of-Care Urinary Diagnostic Platform?
- 2. How does the Inhalable Point-Of-Care Urinary Diagnostic Platform work?
- 3. What are the benefits of this diagnostic platform?
- 4. What components are included in the PATROL system?
- 5. Which proteases are targeted by the ABNs?
- 6. How sensitive is the Lateral Flow Assay (LFA)?
- 7. What clinical applications are suited for the platform?
- 8. How accurate is the inhalable diagnostic platform?
- 9. Where can I find more information about this platform?
- 10. Is the platform safe for regular use?
1. What is the Inhalable Point-of-Care Urinary Diagnostic Platform?
The inhalable point-of-care urinary diagnostic platform, such as PATROL (point-of-care aerosolizable nanosensors with tumor-responsive oligonucleotide barcodes), is a non-invasive method for early lung cancer detection. According to research published in Science Advances, this approach integrates activity-based nanosensors (ABNs) delivered via inhalation with a paper-based lateral flow assay (LFA) for rapid urinary analysis.
1.1. Key Components of the Platform
The platform consists of three primary components:
- Activity-Based Nanosensors (ABNs): These nanosensors are designed to respond to tumor-associated proteases, releasing synthetic DNA reporters.
- Portable Inhalation Unit: This unit ensures efficient delivery of the ABNs to the lungs.
- Multiplexable Paper-Based LFA: This assay allows for rapid, point-of-care detection of the DNA reporters in urine samples.
1.2. How Does It Work?
The process involves the following steps:
- Inhalation: The patient inhales the aerosolized ABNs.
- Targeting: The ABNs reach the lungs, where they interact with tumor-associated proteases.
- Release: Proteases cleave the ABNs, releasing synthetic DNA barcodes.
- Excretion: The DNA barcodes are excreted in the urine.
- Detection: The LFA is used to detect and quantify the DNA barcodes in the urine sample.
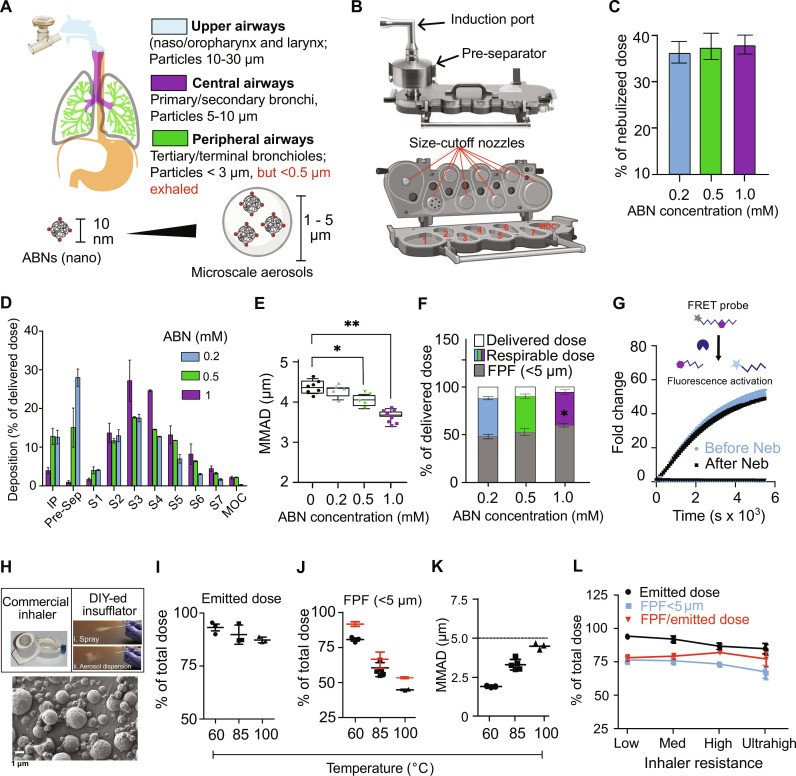 Human adult airways and lung geometry, with predicted aerosol deposition with respect to particle size
Human adult airways and lung geometry, with predicted aerosol deposition with respect to particle size
2. What are the Benefits of Using an Inhalable Diagnostic Platform?
The inhalable diagnostic platform offers several advantages over traditional diagnostic methods, including non-invasive diagnostics, protease dysregulation and rapid results.
2.1. Non-Invasive
Unlike traditional methods such as biopsies or CT scans, the inhalable diagnostic platform is non-invasive, reducing patient discomfort and risk.
2.2. Rapid Results
The LFA provides results within 20 minutes, enabling quick decision-making and timely intervention.
2.3. Accessibility
The point-of-care nature of the platform makes it accessible in resource-limited settings where advanced medical infrastructure may be lacking.
2.4. Cost-Effectiveness
By eliminating the need for expensive equipment and trained personnel, the platform reduces the overall cost of lung cancer screening.
2.5. Early Detection
The platform is designed to detect early-stage tumors, increasing the chances of successful treatment and improving patient outcomes.
3. How Does the PATROL System Work?
The PATROL system integrates three key modules to achieve early lung cancer detection at the point of care.
3.1. Activity-Based Nanosensors (ABNs)
ABNs are designed to target and respond to specific proteases associated with lung cancer. These nanosensors are composed of:
- PEG Nanoscaffold: An eight-arm polyethylene glycol (PEG) nanoscaffold provides structural support.
- Protease Substrates: Tandem peptides containing matrix metalloproteinase (MMP) substrates are attached to the scaffold.
- DNA Barcodes: Synthetic DNA reporters are released upon cleavage by proteases.
3.2. Inhalation Unit
The inhalation unit ensures efficient delivery of the ABNs to the lungs. Key features include:
- Nebulization: A vibrating mesh nebulizer aerosolizes the ABNs into micro-sized particles.
- Aerodynamic Performance: The particle size is optimized for deposition in the peripheral lung, where lung cancer is predominantly located.
3.3. Lateral Flow Assay (LFA)
The LFA enables rapid, point-of-care detection of the DNA barcodes in urine samples. The process involves:
- Urine Sample Application: The urine sample is applied to the conjugate pad of the LFA strip.
- DNA Barcode Labeling: Europium (Eu)-labeled Neutravidin binds to the biotinylated DNA barcodes.
- Hybridization: Labeled DNA barcodes hybridize with capture sequences on the test strip.
- Detection: A POC reader quantifies the fluorescence signal at each test line.
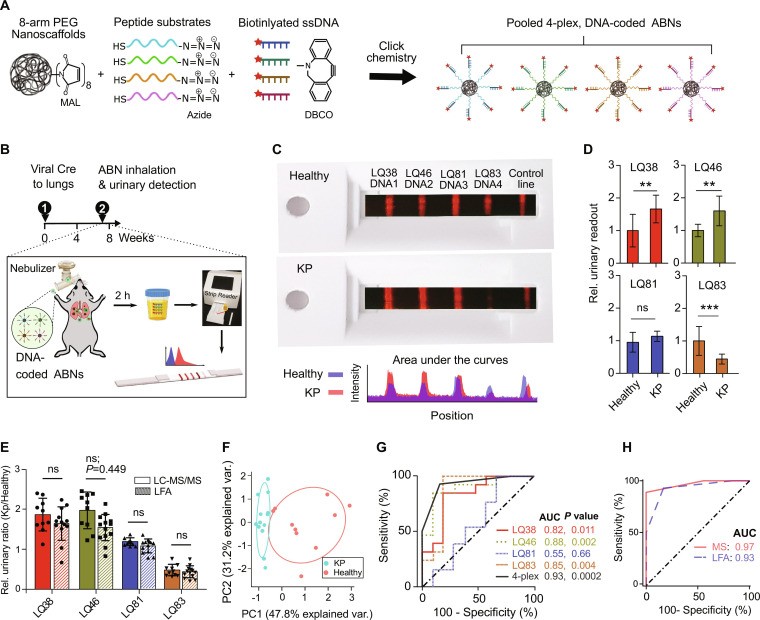 Lateral flow assay (LFA) for the direct multiplexed detection of urinary DNA barcodes at room temperature
Lateral flow assay (LFA) for the direct multiplexed detection of urinary DNA barcodes at room temperature
4. What is the Scientific Evidence Supporting the Platform?
Research published in Science Advances provides strong evidence supporting the efficacy of the inhalable point-of-care urinary diagnostic platform.
4.1. In Vitro Studies
In vitro studies demonstrated the selectivity and sensitivity of the ABNs to target proteases associated with early-stage lung adenocarcinoma.
4.2. In Vivo Studies
In vivo studies using an autochthonous lung adenocarcinoma mouse model showed that the platform could detect early-stage tumors with high sensitivity and specificity. The study found:
- Efficient Lung Delivery: Nebulized ABNs were efficiently delivered to the lungs.
- Accurate Detection: The LFA accurately quantified DNA barcodes in urine samples.
- High Diagnostic Performance: The platform achieved an area under the curve (AUC) of 0.93 in discriminating tumor-bearing mice from healthy controls.
4.3. Safety Studies
Safety studies indicated that the inhalable ABNs were non-toxic and non-immunogenic, supporting their potential for clinical translation.
5. What Proteases are Targeted by the ABNs?
The ABNs are designed to target proteases that are dysregulated in early-stage lung adenocarcinoma. According to the Science Advances study, key proteases include:
- Matrix Metalloproteinases (MMPs): These proteases play a role in tumor progression and are often overexpressed in lung cancer.
- Serine Proteases: These proteases are involved in various biological processes, including inflammation and immune response.
- Cysteine Proteases: These proteases are involved in intracellular protein degradation and have been implicated in cancer development.
5.1. Specific Substrates Used
The ABNs incorporate specific peptide substrates that are selectively cleaved by these proteases. Examples include:
- PLQ38: Sensitive to serine proteases such as PRSS, HGFAC, and F2.
- PLQ46: Also sensitive to serine proteases.
- PLQ81: A metalloprotease substrate.
- PLQ83: Sensitive to serine proteases.
6. How Does the Lateral Flow Assay (LFA) Work in Detail?
The lateral flow assay (LFA) is a crucial component of the inhalable point-of-care urinary diagnostic platform, providing a rapid and multiplexable method for detecting synthetic DNA barcodes in urine samples.
6.1. Components of the LFA Strip
The LFA strip consists of several key components:
- Sample Pad: Where the urine sample is applied.
- Conjugate Pad: Contains europium (Eu)-labeled Neutravidin, which binds to the biotinylated DNA barcodes.
- Nitrocellulose Membrane: The main reaction area where DNA barcodes hybridize with capture sequences.
- Test Lines: Immobilized capture probes specific to each DNA barcode.
- Control Line: Contains biotinylated bovine serum albumin (BSA) to ensure the assay is working correctly.
- Wicking Pad: Absorbs excess liquid and facilitates the flow of the sample.
6.2. Step-by-Step Process
- Sample Application: The urine sample is applied to the sample pad.
- Labeling: As the sample flows through the conjugate pad, the Eu-labeled Neutravidin binds to the biotinylated DNA barcodes.
- Migration: The labeled DNA barcodes migrate along the nitrocellulose membrane.
- Hybridization: The DNA barcodes hybridize with their complementary capture sequences at the test lines.
- Detection: A portable fluorescent reader quantifies the fluorescence intensity at each test line, indicating the concentration of each DNA barcode in the urine sample.
6.3. Multiplexing Capability
The LFA is designed to detect multiple DNA barcodes simultaneously, allowing for a more comprehensive assessment of protease activity. This multiplexing capability is achieved through the spatial printing of individual capture DNA lines on the nitrocellulose membrane.
7. What are the Potential Clinical Applications?
The inhalable point-of-care urinary diagnostic platform has several potential clinical applications in lung cancer detection and management.
7.1. Early Detection of Lung Cancer
The primary application is the early detection of lung cancer, particularly in high-risk individuals or those with limited access to traditional screening methods.
7.2. Monitoring Treatment Response
The platform can be used to monitor the response to lung cancer treatment by tracking changes in protease activity and DNA barcode levels in urine samples.
7.3. Risk Stratification
The platform may help stratify the risk of lung cancer development in individuals with suspicious lung nodules or other risk factors.
7.4. Screening in Resource-Limited Settings
The point-of-care nature of the platform makes it suitable for screening in resource-limited settings where advanced medical infrastructure is lacking.
7.5. Personalized Medicine
The platform could be used to personalize lung cancer treatment by identifying specific protease signatures and tailoring therapy accordingly.
8. What are the Limitations of the Platform?
While the inhalable point-of-care urinary diagnostic platform offers numerous advantages, it also has some limitations that need to be addressed.
8.1. Sensitivity and Specificity
Although the platform has shown high sensitivity and specificity in preclinical studies, further validation is needed to confirm its performance in diverse clinical populations.
8.2. Standardization
Standardizing the platform and ensuring consistent performance across different settings and operators is crucial for its widespread adoption.
8.3. Regulatory Approval
Obtaining regulatory approval for clinical use may be challenging, as the platform involves novel technologies and diagnostic approaches.
8.4. Patient Compliance
Ensuring patient compliance with the inhalation protocol and urine sample collection is essential for accurate results.
8.5. Cost and Reimbursement
The cost of the platform and its reimbursement by healthcare providers may affect its accessibility and adoption.
9. What Future Research is Needed?
Further research is needed to optimize the performance and expand the clinical applications of the inhalable point-of-care urinary diagnostic platform.
9.1. Clinical Validation
Conducting large-scale clinical trials to validate the platform’s sensitivity, specificity, and clinical utility in diverse populations.
9.2. Optimization of ABNs
Optimizing the design and targeting of ABNs to improve their sensitivity and specificity for different subtypes of lung cancer.
9.3. Expansion of Multiplexing Capability
Expanding the multiplexing capability of the LFA to detect a wider range of DNA barcodes and protease signatures.
9.4. Integration with Digital Health Technologies
Integrating the platform with digital health technologies, such as smartphones and wearable devices, to enable remote monitoring and data analysis.
9.5. Cost-Effectiveness Analysis
Conducting cost-effectiveness analyses to assess the economic impact of the platform and its potential to reduce healthcare costs.
10. What are the Implications for the Automotive Industry?
While the inhalable point-of-care urinary diagnostic platform is primarily focused on medical applications, there are potential implications for the automotive industry.
10.1. Sensor Technology
The sensor technology used in the platform, such as the ABNs and LFA, could be adapted for use in automotive diagnostics. For example, sensors could be developed to detect pollutants or other harmful substances in vehicle emissions.
10.2. Point-of-Care Diagnostics
The point-of-care concept could be applied to automotive maintenance, enabling mechanics to quickly diagnose vehicle problems on-site without the need for extensive equipment.
10.3. Materials Science
The materials science involved in the development of the platform, such as the PEG nanoscaffolds and DNA barcodes, could inspire new materials for automotive applications.
10.4. Air Quality Monitoring
The inhalation technology could be used to monitor air quality inside vehicles, ensuring passenger safety and comfort.
10.5. Health and Safety
The health and safety aspects of the platform could inform the development of new safety features in vehicles, such as sensors to detect driver fatigue or impairment.
The inhalable point-of-care urinary diagnostic platform represents a significant advancement in early lung cancer detection, offering a non-invasive, rapid, and accessible alternative to traditional methods. By integrating modular inhalable synthetic biomarkers and multiplexable LFAs, this platform holds great clinical potential for improving patient outcomes and reducing healthcare costs. At CAR-TOOL.EDU.VN, we are committed to providing you with the latest information on innovative technologies like this, helping you stay informed and make better decisions.
Interested in learning more about cutting-edge diagnostic tools and their potential applications? Contact us today at +1 (641) 206-8880 or visit our website at CAR-TOOL.EDU.VN to explore our comprehensive resources and expert insights. Our address is 456 Elm Street, Dallas, TX 75201, United States. Let CAR-TOOL.EDU.VN be your trusted source for the latest advancements in automotive and medical technology.
FAQ: Inhalable Point-Of-Care Urinary Diagnostic Platform
1. What is the Inhalable Point-Of-Care Urinary Diagnostic Platform?
It is a non-invasive diagnostic tool for early lung cancer detection, using inhaled nanosensors and a rapid urine test.
2. How does the Inhalable Point-Of-Care Urinary Diagnostic Platform work?
The platform delivers activity-based nanosensors (ABNs) to the lungs, which release DNA barcodes excreted in urine and detected by a lateral flow assay (LFA).
3. What are the benefits of this diagnostic platform?
The benefits include being non-invasive, providing rapid results, accessibility in resource-limited settings, cost-effectiveness, and early detection capabilities.
4. What components are included in the PATROL system?
The PATROL system includes activity-based nanosensors (ABNs), a portable inhalation unit, and a multiplexable paper-based lateral flow assay (LFA).
5. Which proteases are targeted by the ABNs?
The ABNs target matrix metalloproteinases (MMPs), serine proteases, and cysteine proteases dysregulated in early-stage lung adenocarcinoma.
6. How sensitive is the Lateral Flow Assay (LFA)?
The Lateral Flow Assay (LFA) has the sensitivity to quantify subnanomolar concentrations of the target.
7. What clinical applications are suited for the platform?
Suited clinical applications include early detection of lung cancer, monitoring treatment response, risk stratification, and screening in resource-limited settings.
8. How accurate is the inhalable diagnostic platform?
It shows the high diagnostic performance to the scale of an area under the curve (AUC) of 0.93 in discriminating tumor-bearing mice from healthy controls.
9. Where can I find more information about this platform?
You can find more information about the platform at CAR-TOOL.EDU.VN, where we provide comprehensive resources and expert insights.
10. Is the platform safe for regular use?
Preclinical studies indicate that the inhalable ABNs are non-toxic and non-immunogenic, supporting their potential for clinical translation.