Point Of Care Molecular Diagnostics Companies are revolutionizing healthcare, offering rapid and accurate testing at the patient’s side. CAR-TOOL.EDU.VN provides insights into these advancements, empowering technicians and shop owners to understand and utilize this technology effectively. Discover how point of care molecular diagnostics improve diagnostic precision and turnaround times, enhancing patient care and streamlining automotive repair diagnostics using cutting-edge tools and methods.
Contents
- 1. Understanding Point of Care Molecular Diagnostics Companies
- 1.1 The Significance of Molecular Diagnostics
- 1.2 Benefits of Point of Care Testing
- 1.3 Applications in Automotive Repair
- 2. Key Players in Point of Care Molecular Diagnostics
- 2.1 Abbott Laboratories
- 2.2 F. Hoffmann-La Roche Ltd.
- 2.3 bioMérieux SA
- 2.4 Danaher Corporation
- 2.5 QIAGEN N.V.
- 3. Technological Advancements Driving Growth
- 3.1 Real-Time PCR (RT-PCR)
- 3.2 Isothermal Nucleic Acid Amplification Technologies (INAAT)
- 3.3 Microfluidics
- 3.4 Multiplexing
- 3.5 Digital PCR (dPCR)
- 4. Applications of Point of Care Molecular Diagnostics
- 4.1 Infectious Disease Diagnostics
- 4.2 Oncology
- 4.3 Genetic Testing
- 4.4 Cardiology
- 5. Market Trends and Future Outlook
- 5.1 Market Growth Drivers
- 5.2 Market Challenges
- 5.3 Future Opportunities
- 5.4 Impact on Automotive Diagnostics
- 6. Case Studies: Successful Point of Care Molecular Diagnostics Companies
- 6.1 Cepheid
- 6.2 Alere (now Abbott)
- 7. Regulatory and Ethical Considerations
- 7.1 Regulatory Requirements
- 7.2 Ethical Considerations
- 8. How CAR-TOOL.EDU.VN Can Help
- 8.1 Detailed Product Information
- 8.2 Comparison Tools
- 8.3 User Reviews and Testimonials
- 8.4 Expert Advice and Recommendations
- 8.5 Contact Us for Personalized Assistance
- 9. Frequently Asked Questions (FAQ)
- 9.1 What is Point of Care Molecular Diagnostics?
- 9.2 What Are the Benefits of Point of Care Molecular Diagnostics?
- 9.3 Which Companies Are Leaders in Point of Care Molecular Diagnostics?
- 9.4 What Technologies Drive Point of Care Molecular Diagnostics?
- 9.5 What Are the Applications of Point of Care Molecular Diagnostics?
- 9.6 What Are the Challenges Facing Point of Care Molecular Diagnostics Companies?
- 9.7 What is the Future Outlook for Point of Care Molecular Diagnostics?
- 9.8 How Can Automotive Repair Shops Benefit from Diagnostic Advancements?
- 9.9 How Can CAR-TOOL.EDU.VN Help Automotive Technicians?
- 9.10 How Can I Contact CAR-TOOL.EDU.VN for Assistance?
- 10. Conclusion: Embracing the Future of Diagnostics
1. Understanding Point of Care Molecular Diagnostics Companies
What are point of care molecular diagnostics companies and why are they important? Point of care molecular diagnostics companies specialize in developing and distributing diagnostic tools that enable rapid molecular testing outside of traditional laboratory settings. These companies are crucial because they provide faster results, quicker treatment decisions, and more accessible healthcare, as noted in a study by the National Institutes of Health (NIH).
1.1 The Significance of Molecular Diagnostics
Molecular diagnostics involves analyzing DNA, RNA, and other biomarkers to detect diseases at their earliest stages. According to the World Health Organization (WHO), molecular diagnostics plays a vital role in identifying infectious diseases, genetic disorders, and cancers. These tests are highly specific and sensitive, offering more accurate results than traditional diagnostic methods.
1.2 Benefits of Point of Care Testing
Point of care testing (POCT) brings molecular diagnostics closer to the patient, reducing turnaround times and improving patient outcomes. Key benefits include:
- Rapid Results: Results are available within minutes to hours, allowing for immediate clinical decisions.
- Accessibility: POCT devices can be used in various settings, including clinics, emergency rooms, and even at home.
- Improved Patient Care: Faster diagnosis leads to quicker treatment and better patient management.
- Reduced Costs: By minimizing the need for centralized labs, POCT can lower healthcare costs.
1.3 Applications in Automotive Repair
While primarily used in healthcare, the principles of rapid, accurate diagnostics can be translated to automotive repair. Just as molecular diagnostics identifies specific pathogens, advanced diagnostic tools can pinpoint the exact cause of vehicle malfunctions, leading to faster and more effective repairs. Imagine diagnosing an engine problem with the same speed and accuracy as diagnosing a viral infection.
2. Key Players in Point of Care Molecular Diagnostics
Who are the leading point of care molecular diagnostics companies and what makes them stand out? Several companies are at the forefront of this rapidly evolving field, each offering unique technologies and solutions. Here are some of the top players:
- Abbott Laboratories (US)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- bioMérieux SA (France)
- Danaher Corporation (US)
- QIAGEN N.V. (Netherlands)
These companies lead the market due to their extensive product portfolios, innovative technologies, and strong global presence, according to a report by MarketsandMarkets.
2.1 Abbott Laboratories
Abbott Laboratories is a global healthcare leader offering a wide range of diagnostic products, including point of care molecular tests. Their ID NOW™ platform provides rapid molecular results for infectious diseases such as influenza, strep A, and COVID-19.
- Key Products: ID NOW™ platform for rapid molecular testing
- Strengths: Extensive product portfolio, strong market presence
- Applications: Infectious disease diagnostics
 Abbott ID NOW platform
Abbott ID NOW platform
2.2 F. Hoffmann-La Roche Ltd.
Roche is a multinational healthcare company that develops and manufactures diagnostic systems and tests. Their cobas® Liat® System offers rapid PCR testing for various infectious diseases, providing results in as little as 20 minutes.
- Key Products: cobas® Liat® System for rapid PCR testing
- Strengths: Innovative technology, comprehensive diagnostic solutions
- Applications: Infectious disease diagnostics, oncology
2.3 bioMérieux SA
bioMérieux is a French multinational biotechnology company specializing in in vitro diagnostics. Their BIOFIRE® FilmArray® system offers rapid syndromic testing, identifying multiple pathogens simultaneously from a single sample.
- Key Products: BIOFIRE® FilmArray® system for syndromic testing
- Strengths: Rapid multiplex testing, broad application range
- Applications: Infectious disease diagnostics
2.4 Danaher Corporation
Danaher Corporation is a diversified technology company that owns several leading diagnostic brands, including Cepheid. Cepheid’s GeneXpert® system is a widely used point of care molecular diagnostics platform that offers rapid and accurate results for infectious diseases, oncology, and other applications.
- Key Products: Cepheid GeneXpert® system for rapid molecular testing
- Strengths: Versatile platform, broad application range
- Applications: Infectious disease diagnostics, oncology
2.5 QIAGEN N.V.
QIAGEN is a Dutch multinational biotechnology company that provides sample preparation and molecular testing solutions. Their QIAstat-Dx® system offers rapid multiplex PCR testing for various infectious diseases, providing results in about one hour.
- Key Products: QIAstat-Dx® system for rapid multiplex PCR testing
- Strengths: Multiplex testing, comprehensive diagnostic solutions
- Applications: Infectious disease diagnostics
3. Technological Advancements Driving Growth
What technological advancements are propelling the growth of point of care molecular diagnostics companies? Several key technologies are driving the expansion of the point of care molecular diagnostics market, according to a report by Grand View Research.
3.1 Real-Time PCR (RT-PCR)
Real-time PCR is a highly sensitive and specific technique used to amplify and quantify DNA or RNA. RT-PCR is widely used in point of care molecular diagnostics for detecting infectious diseases, monitoring viral loads, and identifying genetic mutations. Its speed and accuracy make it an essential tool for rapid diagnosis.
3.2 Isothermal Nucleic Acid Amplification Technologies (INAAT)
INAAT methods amplify nucleic acids at a constant temperature, eliminating the need for thermal cycling. This simplifies the testing process and reduces the size and complexity of diagnostic devices. Examples of INAAT technologies include loop-mediated isothermal amplification (LAMP) and transcription-mediated amplification (TMA).
3.3 Microfluidics
Microfluidics involves manipulating small volumes of fluids within microchannels. This technology enables the development of miniaturized, integrated diagnostic devices that can perform sample preparation, amplification, and detection on a single chip. Microfluidic devices offer rapid turnaround times, reduced reagent consumption, and improved portability.
3.4 Multiplexing
Multiplexing allows for the simultaneous detection of multiple targets in a single assay. This is particularly useful for syndromic testing, where multiple pathogens can cause similar symptoms. Multiplex assays can quickly identify the causative agent, enabling targeted treatment decisions.
3.5 Digital PCR (dPCR)
Digital PCR is a highly precise method for quantifying nucleic acids. dPCR involves partitioning a sample into thousands of individual reactions, each containing either zero or one target molecule. By counting the number of positive reactions, dPCR can accurately quantify the target nucleic acid without the need for calibration curves.
4. Applications of Point of Care Molecular Diagnostics
How are point of care molecular diagnostics companies improving healthcare outcomes? Point of care molecular diagnostics has a wide range of applications across various healthcare settings.
4.1 Infectious Disease Diagnostics
Infectious disease diagnostics is one of the primary applications of point of care molecular diagnostics. Rapid and accurate detection of pathogens such as viruses, bacteria, and fungi is crucial for timely treatment and infection control.
- Respiratory Diseases: Rapid detection of influenza, RSV, and COVID-19
- Sexually Transmitted Diseases (STDs): Diagnosis of chlamydia, gonorrhea, and trichomoniasis
- Hospital-Acquired Infections (HAIs): Detection of MRSA, C. difficile, and other HAIs
- Hepatitis: Diagnosis and monitoring of hepatitis B and C
4.2 Oncology
Point of care molecular diagnostics plays an increasingly important role in oncology, enabling rapid detection of cancer biomarkers and monitoring treatment response.
- Liquid Biopsies: Detection of circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA)
- Personalized Medicine: Identification of genetic mutations to guide targeted therapy decisions
- Minimal Residual Disease (MRD) Monitoring: Detection of residual cancer cells after treatment
4.3 Genetic Testing
Point of care molecular diagnostics can be used for rapid genetic testing, providing information about inherited diseases, drug metabolism, and other genetic traits.
- Pharmacogenomics: Identification of genetic variations that affect drug response
- Inherited Disease Screening: Detection of genetic mutations associated with inherited diseases
- Carrier Screening: Identification of individuals who carry a genetic mutation but do not have the disease
4.4 Cardiology
In cardiology, point of care molecular diagnostics can be used to detect biomarkers associated with heart disease and guide treatment decisions.
- Myocardial Infarction: Detection of cardiac troponin and other biomarkers
- Heart Failure: Monitoring of BNP and NT-proBNP levels
- Anticoagulation Management: Monitoring of warfarin and other anticoagulants
5. Market Trends and Future Outlook
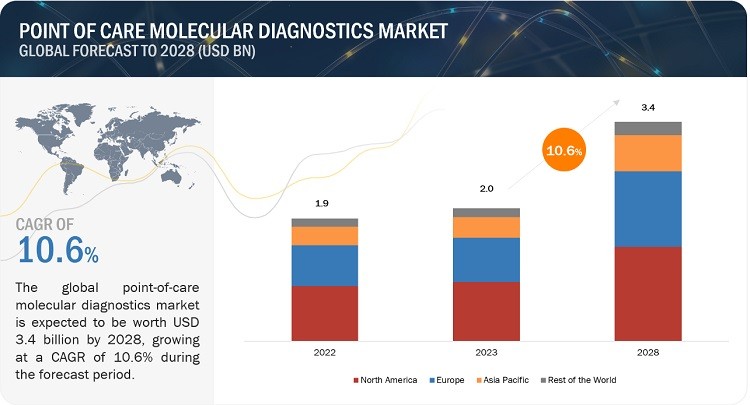
What are the current market trends and what does the future hold for point of care molecular diagnostics companies? The global point of care molecular diagnostics market is experiencing rapid growth, driven by technological advancements, increasing demand for rapid diagnostics, and the rising prevalence of infectious diseases and cancer, according to a report by MarketsandMarkets.
5.1 Market Growth Drivers
- Increasing Incidence of Infectious Diseases: The rising prevalence of infectious diseases such as COVID-19, influenza, and STDs is driving demand for rapid and accurate diagnostic tests.
- Technological Advancements: Advances in molecular diagnostics technologies such as PCR, INAAT, and microfluidics are enabling the development of more sensitive, specific, and user-friendly point of care tests.
- Growing Demand for Personalized Medicine: The increasing focus on personalized medicine is driving demand for molecular diagnostics tests that can identify genetic mutations and guide targeted therapy decisions.
- Rising Healthcare Expenditure: Increasing healthcare expenditure in both developed and developing countries is supporting the growth of the point of care molecular diagnostics market.
5.2 Market Challenges
- Regulatory Hurdles: The development and commercialization of point of care molecular diagnostics tests are subject to strict regulatory requirements, which can be time-consuming and costly.
- Reimbursement Issues: Inadequate reimbursement policies for point of care molecular diagnostics tests can limit their adoption and market growth.
- Competition from Alternative Technologies: Point of care molecular diagnostics faces competition from alternative diagnostic technologies such as rapid antigen tests and serological assays.
- Lack of Infrastructure: The lack of adequate infrastructure in some developing countries can hinder the adoption of point of care molecular diagnostics tests.
5.3 Future Opportunities
- Emerging Markets: Emerging markets such as India, China, and Brazil offer significant growth opportunities for point of care molecular diagnostics companies due to their large populations, increasing healthcare expenditure, and rising prevalence of infectious diseases.
- New Applications: Point of care molecular diagnostics is expanding into new application areas such as oncology, genetic testing, and cardiology, creating new growth opportunities for market players.
- Home-Based Testing: The development of user-friendly point of care molecular diagnostics tests for home-based testing is expected to drive market growth in the coming years.
- Integration with Digital Health Technologies: The integration of point of care molecular diagnostics with digital health technologies such as telemedicine and mobile health is expected to improve patient access to diagnostic services and enhance disease management.
5.4 Impact on Automotive Diagnostics
The advancements in point of care molecular diagnostics offer valuable lessons for the automotive repair industry. By adopting similar principles of rapid, accurate diagnostics, auto repair shops can:
- Improve Diagnostic Accuracy: Advanced diagnostic tools can pinpoint the exact cause of vehicle malfunctions, reducing the need for trial-and-error repairs.
- Reduce Turnaround Times: Faster diagnostics lead to quicker repairs, improving customer satisfaction and shop efficiency.
- Enhance Customer Communication: Accurate diagnostic results allow for clear and transparent communication with customers about the necessary repairs and associated costs.
- Increase Revenue: Efficient diagnostics and repairs can increase the number of vehicles serviced, boosting overall revenue.
6. Case Studies: Successful Point of Care Molecular Diagnostics Companies
What are some real-world examples of successful point of care molecular diagnostics companies? Several companies have achieved significant success in the point of care molecular diagnostics market through innovation, strategic partnerships, and effective commercialization.
6.1 Cepheid
Cepheid is a leading molecular diagnostics company that develops, manufactures, and markets automated systems and tests for rapid detection of infectious diseases. Their GeneXpert® system is a widely used point of care molecular diagnostics platform that offers rapid and accurate results for a variety of applications.
- Success Factors:
- Innovative Technology: The GeneXpert® system offers a fully integrated, automated solution for sample preparation, amplification, and detection.
- Broad Application Range: The GeneXpert® system can be used for a wide range of applications, including infectious disease diagnostics, oncology, and genetic testing.
- Strategic Partnerships: Cepheid has established strategic partnerships with leading healthcare organizations and government agencies to expand the reach of their products.
- Impact: Cepheid’s GeneXpert® system has revolutionized infectious disease diagnostics, enabling rapid and accurate detection of pathogens in a variety of settings.
6.2 Alere (now Abbott)
Alere, now part of Abbott, was a leading provider of point of care diagnostics products for infectious diseases, cardiology, and toxicology. Their Alere i platform offered rapid molecular testing for influenza A & B and RSV.
- Success Factors:
- User-Friendly Design: The Alere i platform was designed for ease of use, requiring minimal training and expertise.
- Rapid Results: The Alere i platform provided results in as little as 15 minutes, enabling rapid clinical decisions.
- Strong Market Presence: Alere had a strong market presence in the point of care diagnostics market, with a wide distribution network and established customer relationships.
- Impact: Alere’s point of care diagnostics products have improved patient care and reduced healthcare costs by enabling rapid and accurate diagnosis of infectious diseases and other conditions.
7. Regulatory and Ethical Considerations
What are the regulatory and ethical considerations for point of care molecular diagnostics companies? The development and commercialization of point of care molecular diagnostics tests are subject to strict regulatory requirements and ethical considerations.
7.1 Regulatory Requirements
- FDA Approval: In the United States, point of care molecular diagnostics tests must be approved by the Food and Drug Administration (FDA) before they can be marketed and sold.
- CLIA Waiver: Point of care molecular diagnostics tests used in CLIA-waived settings must meet certain criteria to ensure their accuracy and reliability.
- CE Marking: In Europe, point of care molecular diagnostics tests must be CE marked to demonstrate that they meet the requirements of the European Union’s Medical Device Directive.
7.2 Ethical Considerations
- Data Privacy: Point of care molecular diagnostics tests generate sensitive patient data, which must be protected from unauthorized access and misuse.
- Informed Consent: Patients must be fully informed about the risks and benefits of point of care molecular diagnostics tests before they undergo testing.
- Accuracy and Reliability: Point of care molecular diagnostics tests must be accurate and reliable to ensure that patients receive appropriate treatment.
- Accessibility: Point of care molecular diagnostics tests should be accessible to all patients, regardless of their socioeconomic status or geographic location.
8. How CAR-TOOL.EDU.VN Can Help
How can CAR-TOOL.EDU.VN assist automotive technicians and shop owners in leveraging diagnostic advancements? At CAR-TOOL.EDU.VN, we understand the importance of staying ahead in the rapidly evolving world of automotive diagnostics. We provide comprehensive resources to help you leverage the latest advancements in diagnostic technology, inspired by the precision and speed of point of care molecular diagnostics.
8.1 Detailed Product Information
We offer detailed specifications, features, and benefits of various automotive diagnostic tools, helping you make informed decisions about the best equipment for your needs. From OBD-II scanners to advanced engine analyzers, our product information is designed to enhance your diagnostic capabilities.
8.2 Comparison Tools
Our comparison tools allow you to easily compare different diagnostic tools side-by-side, evaluating their features, price points, and user reviews. This ensures you find the tool that best fits your budget and diagnostic requirements.
8.3 User Reviews and Testimonials
Benefit from the experiences of other automotive technicians and shop owners through our user reviews and testimonials. Gain insights into the real-world performance of diagnostic tools and learn how they can improve your shop’s efficiency and accuracy.
8.4 Expert Advice and Recommendations
Our team of automotive diagnostic experts provides valuable advice and recommendations to help you optimize your diagnostic processes. We offer guidance on selecting the right tools, interpreting diagnostic data, and implementing best practices for automotive repair.
8.5 Contact Us for Personalized Assistance
Do you have questions or need personalized assistance in selecting the right diagnostic tools for your shop? Contact us today via WhatsApp at +1 (641) 206-8880 or visit our website at CAR-TOOL.EDU.VN. Our team is ready to help you enhance your diagnostic capabilities and improve your shop’s performance.
9. Frequently Asked Questions (FAQ)
What are some common questions about point of care molecular diagnostics companies? Here are some frequently asked questions to help you better understand this rapidly evolving field.
9.1 What is Point of Care Molecular Diagnostics?
Point of care molecular diagnostics involves performing molecular tests near the patient, providing rapid results for timely clinical decisions.
9.2 What Are the Benefits of Point of Care Molecular Diagnostics?
Benefits include rapid results, improved accessibility, enhanced patient care, and reduced healthcare costs.
9.3 Which Companies Are Leaders in Point of Care Molecular Diagnostics?
Leading companies include Abbott Laboratories, F. Hoffmann-La Roche Ltd., bioMérieux SA, Danaher Corporation, and QIAGEN N.V.
9.4 What Technologies Drive Point of Care Molecular Diagnostics?
Key technologies include RT-PCR, INAAT, microfluidics, multiplexing, and digital PCR.
9.5 What Are the Applications of Point of Care Molecular Diagnostics?
Applications include infectious disease diagnostics, oncology, genetic testing, and cardiology.
9.6 What Are the Challenges Facing Point of Care Molecular Diagnostics Companies?
Challenges include regulatory hurdles, reimbursement issues, competition from alternative technologies, and lack of infrastructure.
9.7 What is the Future Outlook for Point of Care Molecular Diagnostics?
The future outlook is positive, with growth opportunities in emerging markets, new applications, home-based testing, and integration with digital health technologies.
9.8 How Can Automotive Repair Shops Benefit from Diagnostic Advancements?
Automotive repair shops can improve diagnostic accuracy, reduce turnaround times, enhance customer communication, and increase revenue by adopting advanced diagnostic tools and techniques.
9.9 How Can CAR-TOOL.EDU.VN Help Automotive Technicians?
CAR-TOOL.EDU.VN provides detailed product information, comparison tools, user reviews, expert advice, and personalized assistance to help automotive technicians leverage diagnostic advancements.
9.10 How Can I Contact CAR-TOOL.EDU.VN for Assistance?
You can contact us via WhatsApp at +1 (641) 206-8880 or visit our website at CAR-TOOL.EDU.VN for personalized assistance with your diagnostic needs.
10. Conclusion: Embracing the Future of Diagnostics
The point of care molecular diagnostics market is transforming healthcare by providing rapid, accurate, and accessible diagnostic solutions. While these advancements are primarily focused on healthcare, the principles of rapid and precise diagnostics can be applied to other industries, including automotive repair. By staying informed about the latest technologies and leveraging resources like CAR-TOOL.EDU.VN, automotive technicians and shop owners can enhance their diagnostic capabilities, improve customer satisfaction, and drive business growth. Contact CAR-TOOL.EDU.VN at 456 Elm Street, Dallas, TX 75201, United States, or via WhatsApp at +1 (641) 206-8880 to explore how you can revolutionize your automotive diagnostics today.
