The Point Of Care Molecular Diagnostics Technology Market is poised for substantial growth, projected to increase from USD 2.0 billion in 2023 to USD 3.4 billion by 2028, according to CAR-TOOL.EDU.VN. This expansion, driven by a CAGR of 10.6%, reflects an escalating need for rapid diagnostic solutions, enhanced healthcare infrastructure, and the innovative use of medical diagnostic equipment and automotive diagnostic tools. Let’s explore the factors influencing this market, the technologies driving its evolution, and the opportunities it presents.
Contents
- 1. Understanding The Point Of Care Molecular Diagnostics Technology Market
- 1.1 What Is Point Of Care Molecular Diagnostics Technology?
- 1.2 What Are The Key Applications Of Point Of Care Molecular Diagnostics Technology?
- 1.3 What Are The Benefits Of Point Of Care Molecular Diagnostics Technology?
- 1.4 What Is The Market Size And Growth Potential Of Point Of Care Molecular Diagnostics Technology?
- 2. Key Market Dynamics
- 2.1 What Are The Market Drivers?
- 2.2 What Are The Market Restraints?
- 2.3 What Are The Market Opportunities?
- 2.4 What Are The Market Challenges?
- 3. Technology Trends Shaping The Market
- 3.1 What Is RT-PCR (Real-Time PCR)?
- 3.2 What Is INAAT (Isothermal Nucleic Acid Amplification Technology)?
- 3.3 What Are Microfluidics?
- 3.4 What Are Multiplex Assays?
- 3.5 What Are Digital PCR (dPCR)?
- 4. Point Of Care Molecular Diagnostics Technology Market Segmentation
- 4.1 What Is The Segmentation Based On Product & Service?
- 4.2 What Is The Segmentation Based On Technology?
- 4.3 What Is The Segmentation Based On Application?
- 4.4 What Is The Segmentation Based On End User?
- 4.5 What Is The Segmentation Based On Region?
- 5. Regional Analysis Of The Point Of Care Molecular Diagnostics Technology Market
- 5.1 North America
- 5.2 Europe
- 5.3 Asia Pacific
- 5.4 Latin America
- 5.5 Middle East & Africa
- 6. Competitive Landscape
- 6.1 Key Players
- 6.2 Market Strategies
- 6.3 Recent Developments
- 7. The Role Of Automotive Diagnostic Tools In Parallel Industries
- 8. Conclusion
- 9. Frequently Asked Questions (FAQ)
- 9.1 What Is The Expected Growth Rate Of The Global Point Of Care Molecular Diagnostics Market From 2023 To 2028?
- 9.2 What Factors Are Driving The Growth Of The Point Of Care Molecular Diagnostics Market?
- 9.3 What Challenges Are Faced By The Point Of Care Molecular Diagnostics Market?
- 9.4 What Opportunities Exist In Emerging Markets For The Point Of Care Molecular Diagnostics Industry?
- 9.5 Which Product Segment Holds The Largest Market Share In The Point Of Care Molecular Diagnostics Market?
- 9.6 What Role Does RT-PCR Technology Play In The Point Of Care Molecular Diagnostics Market?
- 9.7 Which Region Dominates The Point Of Care Molecular Diagnostics Market?
- 9.8 What Is The Impact Of The Increasing Incidence Of Infectious Diseases On The Point Of Care Molecular Diagnostics Market?
- 9.9 Which End-User Segment Accounted For The Largest Share Of The Point Of Care Molecular Diagnostics Market In 2022?
- 9.10 What Are The Recent Developments In The Point Of Care Molecular Diagnostics Market?
- Contact Us For More Information
1. Understanding The Point Of Care Molecular Diagnostics Technology Market
The point of care molecular diagnostics technology market is undergoing a significant transformation, driven by the increasing need for rapid and accurate diagnostic solutions. Molecular diagnostics, which involves the detection and analysis of DNA, RNA, and other biomarkers, is becoming increasingly important in various clinical settings. This market is not only benefiting healthcare but also drawing parallels with the automotive industry, where diagnostic tools like automotive diagnostic tools play a crucial role in maintaining and repairing vehicles efficiently.
1.1 What Is Point Of Care Molecular Diagnostics Technology?
Point of care molecular diagnostics refers to diagnostic testing performed near the patient, providing rapid results that enable quick clinical decisions. According to a report by MarketsandMarkets, the global point of care molecular diagnostics market is expected to grow from $2.0 billion in 2023 to $3.4 billion by 2028, exhibiting a CAGR of 10.6%.
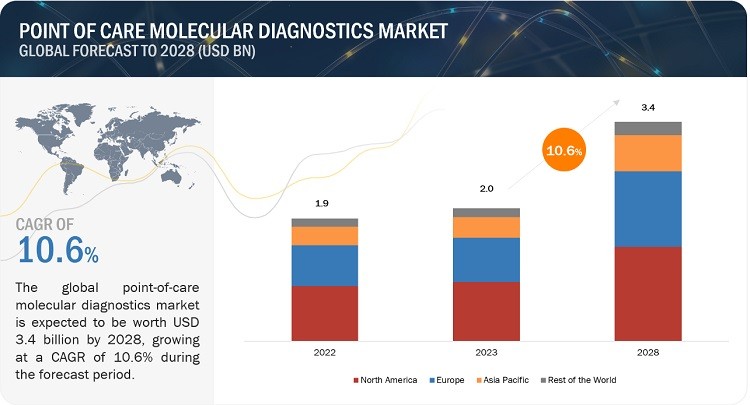 Point of Care Molecular Diagnostics Market Analysis
Point of Care Molecular Diagnostics Market Analysis
Alt: Market analysis showing the projected growth of the Point of Care Molecular Diagnostics Market.
This growth is primarily driven by the rising incidence of infectious diseases, cancers, and the increasing focus on improving healthcare facilities and infrastructure. These technologies allow healthcare providers to quickly diagnose conditions, monitor treatment efficacy, and implement appropriate interventions in a timely manner.
1.2 What Are The Key Applications Of Point Of Care Molecular Diagnostics Technology?
The applications of point of care molecular diagnostics technology are broad and impactful, spanning multiple areas of healthcare.
- Infectious Disease Diagnostics: Rapid detection of pathogens like influenza, respiratory syncytial virus (RSV), and sexually transmitted infections (STIs). A study published in the Journal of Clinical Microbiology highlighted the effectiveness of point-of-care PCR assays in diagnosing influenza, reducing the time to diagnosis from days to hours.
- Cancer Diagnostics: Early detection and monitoring of cancer biomarkers to guide treatment decisions. Research from the National Cancer Institute emphasizes the potential of molecular diagnostics in personalized cancer therapy.
- Chronic Disease Management: Monitoring chronic conditions such as diabetes and cardiovascular diseases through biomarker analysis.
- Pharmacogenomics: Tailoring drug therapies based on an individual’s genetic makeup to optimize treatment efficacy and minimize adverse effects.
- Emergency and Critical Care: Rapid diagnosis of critical conditions in emergency rooms and intensive care units, enabling timely interventions.
1.3 What Are The Benefits Of Point Of Care Molecular Diagnostics Technology?
The benefits of point of care molecular diagnostics technology are numerous and significant.
- Rapid Results: Tests performed near the patient provide results in minutes to hours, compared to days for traditional lab-based tests.
- Improved Clinical Decision-Making: Quick access to diagnostic information enables healthcare providers to make informed decisions promptly.
- Enhanced Patient Outcomes: Early and accurate diagnosis leads to timely treatment, improving patient outcomes and reducing healthcare costs.
- Reduced Hospital Stays: Faster diagnosis and treatment can reduce the length of hospital stays, freeing up resources and improving patient flow.
- Increased Accessibility: Point of care testing can be deployed in remote and resource-limited settings, improving access to diagnostic services for underserved populations.
1.4 What Is The Market Size And Growth Potential Of Point Of Care Molecular Diagnostics Technology?
The global point of care molecular diagnostics market is substantial and growing rapidly. According to MarketsandMarkets, the market is projected to grow from USD 2.0 billion in 2023 to USD 3.4 billion by 2028, representing a CAGR of 10.6%. This growth is driven by:
- Rising Incidence of Infectious Diseases: The increasing prevalence of infectious diseases, such as respiratory infections, STIs, and hospital-acquired infections, is driving demand for rapid diagnostic solutions.
- Advancements in Technology: Innovations in molecular diagnostics technologies, such as PCR, isothermal amplification, and microfluidics, are improving the speed, accuracy, and ease of use of point of care testing.
- Increasing Healthcare Expenditure: The growing investment in healthcare infrastructure and diagnostic services is supporting the expansion of the market.
- Shift Towards Decentralized Testing: The trend towards decentralized testing, where diagnostic services are brought closer to the patient, is fueling demand for point of care solutions.
 Point of Care Molecular Diagnostics Market Growth Forecast
Point of Care Molecular Diagnostics Market Growth Forecast
Alt: Forecast showing the projected market growth of the Point of Care Molecular Diagnostics Market.
2. Key Market Dynamics
Several key dynamics are shaping the point of care molecular diagnostics technology market, influencing its growth and evolution.
2.1 What Are The Market Drivers?
- Growing Incidence of Infectious Diseases and Cancer: The rising prevalence of infectious diseases and cancer is a primary driver for the point of care molecular diagnostics market. The need for rapid and accurate diagnosis to initiate timely treatment is crucial.
- Increasing Focus on Decentralized Testing: Decentralized testing, bringing diagnostic services closer to the patient, is gaining traction. This trend is particularly important in remote and resource-limited settings.
- Advancements in Molecular Diagnostics Technologies: Technological advancements in PCR, isothermal amplification, and microfluidics are improving the speed, accuracy, and ease of use of point of care testing.
- Rising Healthcare Expenditure: Increasing investments in healthcare infrastructure and diagnostic services are supporting the growth of the point of care molecular diagnostics market.
2.2 What Are The Market Restraints?
- Unfavorable Reimbursement Settings: Inadequate reimbursement policies can hinder market growth. Diagnostic companies face challenges in obtaining payments from Medicare and private health insurers for their tests.
- Regulatory Challenges: Stringent regulatory requirements for diagnostic products can delay market entry and increase compliance costs.
- High Cost of Instruments and Assays: The high cost of molecular diagnostic instruments and assays can limit their adoption, particularly in resource-limited settings.
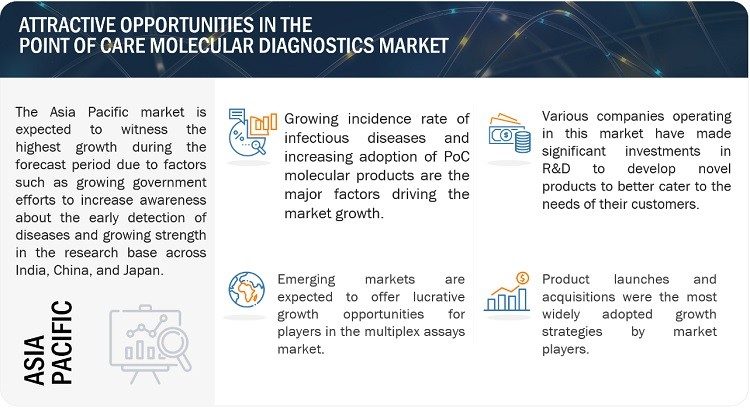
2.3 What Are The Market Opportunities?
- Growth Prospects in Emerging Countries: Emerging economies like India, South Korea, Brazil, and Mexico offer substantial growth prospects. These opportunities stem from factors such as low regulatory barriers, healthcare infrastructure advancements, expanding patient populations, and increasing healthcare expenditure.
- Development of New Applications: Expanding the applications of point of care molecular diagnostics in areas such as chronic disease management, pharmacogenomics, and personalized medicine can drive market growth.
- Integration of Digital Health Technologies: Combining point of care molecular diagnostics with digital health technologies, such as telemedicine and mobile health, can improve access to diagnostic services and enhance patient care.
2.4 What Are The Market Challenges?
- Establishment of Alternative Technologies: The emergence of alternative technologies, including rapid antigen tests, presents a potential obstacle to the growth of the market. These tests are often cheaper and provide faster results.
- Data Management and Connectivity: Managing and integrating the data generated by point of care molecular diagnostic devices can be challenging. Ensuring seamless connectivity with electronic health records (EHRs) and other healthcare IT systems is crucial.
- Quality Control and Accuracy: Maintaining the quality and accuracy of point of care testing is essential. Implementing robust quality control measures and training programs for healthcare providers is necessary to ensure reliable results.
3. Technology Trends Shaping The Market
Several technology trends are shaping the point of care molecular diagnostics technology market, driving innovation and improving the performance of diagnostic solutions.
3.1 What Is RT-PCR (Real-Time PCR)?
RT-PCR is a widely used technology in molecular diagnostics due to its ease of use, cost-effectiveness, and prompt turnaround time. This method is used to amplify and quantify DNA or RNA targets, making it ideal for detecting infectious diseases and monitoring viral loads.
3.2 What Is INAAT (Isothermal Nucleic Acid Amplification Technology)?
INAAT is an alternative amplification method that does not require thermal cycling, making it faster and more suitable for point of care applications. Various INAAT techniques, such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA), are gaining popularity.
3.3 What Are Microfluidics?
Microfluidics involves the manipulation of small volumes of fluids in miniaturized devices. These devices enable rapid and automated sample processing, reducing the time and cost of molecular diagnostics. Microfluidic-based point of care systems are increasingly used for infectious disease testing and cancer diagnostics.
3.4 What Are Multiplex Assays?
Multiplex assays allow the simultaneous detection of multiple targets in a single test. This approach is particularly useful for diagnosing complex infections and identifying multiple pathogens in a single sample.
3.5 What Are Digital PCR (dPCR)?
Digital PCR is a highly sensitive method for quantifying nucleic acids. dPCR involves partitioning a sample into thousands of individual reactions, allowing for precise and accurate quantification of target molecules.
4. Point Of Care Molecular Diagnostics Technology Market Segmentation
The point of care molecular diagnostics market can be segmented based on product type, technology, application, end-user, and region.
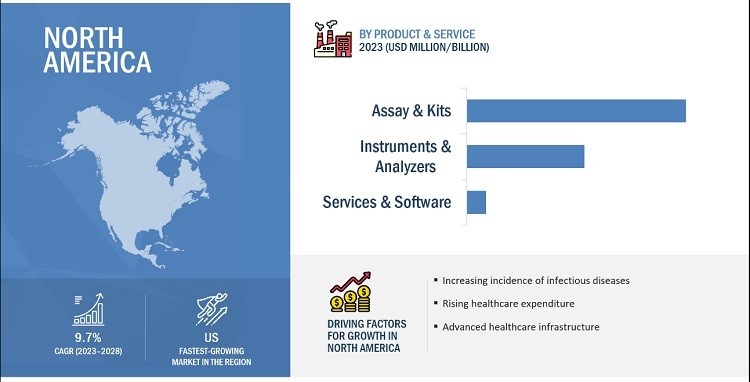
4.1 What Is The Segmentation Based On Product & Service?
- Assays & Kits: These include reagents, controls, and consumables used in molecular diagnostic tests.
- Instruments & Analyzers: These are the devices used to perform molecular diagnostic tests.
- Software & Services: This includes software solutions for data analysis and management, as well as services such as training and support.
According to market research, the assays & kits segment accounted for the largest share of the market in 2022 due to the frequent purchase of these products.
4.2 What Is The Segmentation Based On Technology?
- RT-PCR: Real-time polymerase chain reaction.
- INAAT: Isothermal nucleic acid amplification technology.
- Other Technologies: Including microarrays, next-generation sequencing (NGS), and digital PCR.
In 2022, the RT-PCR segment accounted for the largest share due to its widespread use and cost-effectiveness.
4.3 What Is The Segmentation Based On Application?
- Respiratory Diseases: Including influenza, RSV, and COVID-19.
- Sexually Transmitted Diseases: Including chlamydia, gonorrhea, and HIV.
- Hospital-Acquired Infections: Including MRSA and C. difficile.
- Cancer: Including early detection and monitoring of cancer biomarkers.
- Hepatitis: Including HBV and HCV.
- Gastrointestinal Disorders: Including C. difficile and norovirus.
- Other Applications: Including pharmacogenomics and personalized medicine.
4.4 What Is The Segmentation Based On End User?
- Physicians’ Offices: Point of care molecular assay & kits and systems are extensively used in physicians’ offices as they provide quick results within 30 minutes. This helps in instant diagnosis which allows to diagnose or monitor a patient’s condition immediately.
- Hospitals & ICUs: Hospitals and intensive care units.
- Research Institutes: Research and academic institutions.
- Other End Users: Including public health laboratories and home healthcare settings.
In 2022, the physicians’ offices segment accounted for the largest share of the market, driven by the need for rapid and accurate diagnostic results in clinical settings.
4.5 What Is The Segmentation Based On Region?
- North America: U.S. and Canada.
- Europe: UK, Germany, France, Italy, and Rest of Europe.
- Asia Pacific: China, Japan, India, and Rest of Asia Pacific.
- Latin America.
- **Middle East & Africa.
North America held the largest share of the global market in 2022, driven by the high burden of infectious diseases and cancers, well-developed healthcare infrastructure, and the growing adoption of advanced technologies.
5. Regional Analysis Of The Point Of Care Molecular Diagnostics Technology Market
The point of care molecular diagnostics market varies significantly across different regions, influenced by factors such as healthcare infrastructure, regulatory policies, and the prevalence of infectious diseases.
5.1 North America
North America is the largest market for point of care molecular diagnostics, driven by:
- High prevalence of infectious diseases and cancers.
- Well-developed healthcare infrastructure.
- Growing adoption of advanced diagnostic technologies.
- Favorable reimbursement policies.
The U.S. is the dominant market in North America, with a strong presence of leading diagnostic companies and research institutions.
5.2 Europe
Europe is the second-largest market, driven by:
- Increasing focus on decentralized testing.
- Rising healthcare expenditure.
- Advancements in molecular diagnostics technologies.
- Stringent regulatory requirements.
Germany, the UK, and France are key markets in Europe, with significant investments in healthcare and research.
5.3 Asia Pacific
Asia Pacific is the fastest-growing market, driven by:
- Expanding patient populations.
- Improving healthcare infrastructure.
- Increasing prevalence of infectious diseases.
- Low regulatory barriers in some countries.
China, Japan, and India are key markets in Asia Pacific, with significant opportunities for market growth.
5.4 Latin America
Latin America is an emerging market, driven by:
- Increasing healthcare expenditure.
- Rising prevalence of infectious diseases.
- Improving access to diagnostic services.
- Limited healthcare infrastructure in some countries.
Brazil and Mexico are key markets in Latin America, with significant potential for market growth.
5.5 Middle East & Africa
The Middle East & Africa is a smaller market, driven by:
- Increasing healthcare expenditure in some countries.
- Rising prevalence of infectious diseases.
- Limited healthcare infrastructure in many countries.
- Political and economic instability in some regions.
Saudi Arabia, the UAE, and South Africa are key markets in the Middle East & Africa, with significant investments in healthcare.
6. Competitive Landscape
The point of care molecular diagnostics market is characterized by intense competition, with a mix of well-established players and emerging companies.
6.1 Key Players
- Abbott Laboratories (US): Abbott is a leading player in the point of care molecular diagnostics market, offering a wide range of diagnostic products and services.
- F. Hoffmann-La Roche Ltd. (Switzerland): Roche is a global leader in diagnostics and pharmaceuticals, with a strong presence in the point of care molecular diagnostics market.
- bioMérieux SA (France): bioMérieux is a leading player in the field of in vitro diagnostics, offering a comprehensive range of diagnostic solutions.
- Danaher Corporation (US): Danaher is a diversified technology company with a strong presence in the life sciences and diagnostics industries.
- QIAGEN N.V. (Netherlands): QIAGEN is a leading provider of sample and assay technologies, with a strong focus on molecular diagnostics.
6.2 Market Strategies
Key strategies adopted by market players include:
- Product Innovation: Developing innovative and differentiated diagnostic solutions.
- Strategic Acquisitions and Partnerships: Acquiring or partnering with other companies to expand product portfolios and geographic reach.
- Geographic Expansion: Expanding into emerging markets to capitalize on growth opportunities.
- Focus on Customer Needs: Developing solutions that meet the specific needs of healthcare providers and patients.
6.3 Recent Developments
- QIAGEN N.V. (Netherlands) launched QIAstat-Dx in Japan with a respiratory panel for syndromic testing in April 2023.
- Biocartis NV (Belgium) launched the Rapid CE-marked IVD Idylla GeneFusion Panel for fast treatment decisions in lung cancer in June 2022.
- bioMérieux SA (France) received De Novo FDA Authorization for its BIOFIRE Joint Infection (JI) Panel in May 2022.
- F. Hoffmann-La Roche Ltd. (Switzerland) acquired TIB Molbiol (Germany) to expand its PCR test portfolio with a wide range of assays for infectious diseases in September 2021.
7. The Role Of Automotive Diagnostic Tools In Parallel Industries
Interestingly, the principles of rapid and accurate diagnostics are not limited to healthcare. In the automotive industry, automotive diagnostic tools are essential for quickly identifying and resolving mechanical and electronic issues. These tools allow mechanics to diagnose problems efficiently, reducing repair times and improving vehicle performance, much like point of care molecular diagnostics improve patient outcomes.
CAR-TOOL.EDU.VN recognizes the importance of both medical and automotive diagnostics in ensuring the health and safety of individuals and the reliability of vehicles. By providing detailed information and resources, CAR-TOOL.EDU.VN aims to empower professionals in both fields with the knowledge they need to excel.
8. Conclusion
The point of care molecular diagnostics technology market is poised for significant growth, driven by the increasing need for rapid and accurate diagnostic solutions, advancements in technology, and the rising prevalence of infectious diseases and cancers. While the market faces challenges such as unfavorable reimbursement settings and regulatory hurdles, the opportunities in emerging markets and the development of new applications offer significant growth potential.
 Point of Care Molecular Diagnostics for Global Health
Point of Care Molecular Diagnostics for Global Health
Alt: Geographic regions showing market dominance for Point of Care Molecular Diagnostics.
As technology continues to evolve and healthcare infrastructure improves, point of care molecular diagnostics will play an increasingly important role in improving patient outcomes and reducing healthcare costs. For professionals seeking reliable and detailed information on both medical and automotive diagnostics, CAR-TOOL.EDU.VN stands as a trusted resource.
9. Frequently Asked Questions (FAQ)
9.1 What Is The Expected Growth Rate Of The Global Point Of Care Molecular Diagnostics Market From 2023 To 2028?
The global point of care molecular diagnostics market is projected to grow from USD 2.0 billion in 2023 to USD 3.4 billion by 2028, at a CAGR of 10.6%, driven by the rising incidence of infectious diseases and the increasing focus on healthcare infrastructure improvement.
9.2 What Factors Are Driving The Growth Of The Point Of Care Molecular Diagnostics Market?
The major drivers of the point of care molecular diagnostics market include the growing incidence of infectious diseases and cancer, advancements in healthcare infrastructure, and the increasing preference for preventive medicine.
9.3 What Challenges Are Faced By The Point Of Care Molecular Diagnostics Market?
One of the main challenges for the point of care molecular diagnostics market is unfavorable reimbursement settings, along with the availability of alternative diagnostic technologies such as antigen tests, which can be cheaper and faster.
9.4 What Opportunities Exist In Emerging Markets For The Point Of Care Molecular Diagnostics Industry?
Emerging economies such as India, South Korea, Brazil, and Mexico offer significant growth opportunities due to their expanding patient populations, improving healthcare infrastructure, and increasing prevalence of infectious diseases.
9.5 Which Product Segment Holds The Largest Market Share In The Point Of Care Molecular Diagnostics Market?
The assays and kits segment held the largest share of the point of care molecular diagnostics market in 2022, driven by the frequent and recurrent usage of these products for diagnostic purposes.
9.6 What Role Does RT-PCR Technology Play In The Point Of Care Molecular Diagnostics Market?
RT-PCR technology accounts for the largest share in the point of care molecular diagnostics market due to its widespread use, cost-effectiveness, and rapid turnaround time in diagnosing various diseases.
9.7 Which Region Dominates The Point Of Care Molecular Diagnostics Market?
North America holds the largest share of the point of care molecular diagnostics market, driven by a high incidence of infectious diseases, advanced healthcare infrastructure, and the growing adoption of molecular diagnostic technologies.
9.8 What Is The Impact Of The Increasing Incidence Of Infectious Diseases On The Point Of Care Molecular Diagnostics Market?
The growing incidence of infectious diseases is a key driver of demand for point of care molecular diagnostics, particularly in respiratory and sexually transmitted diseases, contributing to market expansion.
9.9 Which End-User Segment Accounted For The Largest Share Of The Point Of Care Molecular Diagnostics Market In 2022?
In 2022, physicians’ offices held the largest share of the market due to the widespread use of point of care molecular diagnostics for quick and efficient patient diagnosis in a clinical setting.
9.10 What Are The Recent Developments In The Point Of Care Molecular Diagnostics Market?
Recent developments include QIAGEN’s launch of QIAstat-Dx in Japan for syndromic testing and bioMérieux’s FDA authorization for its BIOFIRE Joint Infection Panel.
Contact Us For More Information
Do you need more information on point of care molecular diagnostics technology or automotive diagnostic tools? Contact CAR-TOOL.EDU.VN today. Our team of experts is ready to provide detailed insights and answer your questions.
Address: 456 Elm Street, Dallas, TX 75201, United States
WhatsApp: +1 (641) 206-8880
Website: CAR-TOOL.EDU.VN
Don’t hesitate—reach out now to explore how our resources can benefit your work and enhance your expertise in diagnostics.